Age-dependent preference in human antibody responses to Streptococcus pneumoniae polypeptide antigens - PubMed (original) (raw)
Comparative Study
Age-dependent preference in human antibody responses to Streptococcus pneumoniae polypeptide antigens
S Lifshitz et al. Clin Exp Immunol. 2002 Feb.
Abstract
Vulnerability to Streptococcus pneumoniae is most pronounced in children. The microbial virulence factors and the features of the host immune response contributing to this phenomenon are not completely understood. In the current study, the humoral immune response to separated Strep. pneumoniae surface proteins and the ability to interfere with Strep. pneumoniae adhesion to cultured epithelial cells were analysed in adults and in children. Sera collected from healthy adults recognized Strep. pneumoniae separated lectin and nonlectin surface proteins in Western blot analysis and inhibited on average 80% of Strep. pneumoniae adhesion to epithelial cells in a concentration-dependent manner. However, sera longitudinally collected from healthy children attending day care centres from 18 months of age and over the course of the following 2 years revealed: (a) development of antibodies to previously unrecognized Strep. pneumoniae surface proteins with age; (b) a quantitative increase in antibody responses, measured by densitometry, towards separated Strep. pneumoniae surface proteins with age; and (c) inhibition of Strep. pneumoniae adhesion to epithelial cells, which was 50% on average at 18 months of age, increased significantly to an average level of 80% inhibition at 42 months of age equalling adult sera inhibitory values. The results obtained in the current study, from the longitudinally collected sera from healthy children with documented repeated Strep. pneumoniae colonization, show that repeated exposures are insufficient to elicit an immune response to Strep. pneumoniae proteins at 18 months of age. This inability to recognize Strep. pneumoniae surface proteins may stem from the inefficiency of T-cell-dependent B-cell responses at this age and/or from the low immunogenicity of the proteins.
Figures
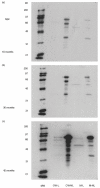
Fig. 1
Antibodies to Strep. pneumoniae surface molecule in randomly chosen healthy adults. Sera from healthy adults were analysed by Western blot analysis for the presence of anti Strep. pneumoniae surface protein antibodies with Strep. pneumoniae fractionated surface molecules. (a) Cell wall (CW) lectin-like molecules. (b) CW proteins that did not adhere to the fetuin column. (c) Membrane (M) lectin-like molecules. (d) Fetuin nonadherent M molecules. MW: molecular weight markers. Lanes 1–8 represent probing with sera from eight different healthy adults. Only bands corresponding to teichoic acid and lipoteichoic acid (around 20 kD) could be identified on Western blots prepared from proteinase K digested CW and M fractions (data not shown).
Fig. 2
Qualitative development and de novo synthesis of antibodies to Strep. pneumoniae surface proteins in children. A representative series of Western blots prepared from Strep. pneumoniae surface molecules and probed with sera collected longitudinally at 18 (a), 30 (b) and 42 (c) months of age from a child attending a day care centre. Lane 1: MW markers; lane 2: cell wall lectin-like (CW-L) proteins; lane 3: cell wall nonlectin (CW-NL) proteins; lane 4: membrane lectin-like (M-L) proteins; lane 5: membrane nonlectin proteins (M-NL).
Fig. 3
Quantitative increase of immune responses to Strep. pneumoniae surface proteins. Serial sera from eight healthy children attending a day care centre were tested for immune responses to Strep. pneumoniae surface proteins using Western blotting. To quantify the increase the blots were prepared from the same Strep. pneumoniae preparation and developed for 2 s (similar increases were obtained with longer exposure time but saturation prevented accurate quantification). TINA software was used for densitometry. An increase between 2 and 107 times was found in 90% of the cases in sera samples obtained at 30 and 42 months of age compared with sera obtained at 18 months of age. ✦ Child #1001; ▪ child #1007; ▴ child #1014; • child #1020; ▵ child #1021; ○ child #1029; ◊ child #1032; □ child #1048.
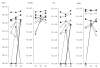
Fig. 4
Inhibition of Strep. pneumoniae adhesion to epithelial cells. Inhibition of Strep. pneumoniae adhesion to HaCat epithelial cells was performed as described in the Methods section with: (a) pooled healthy adult sera; (b) immunoglobulin fraction from adult sera – it should be noted that the serum fraction devoided of immunoglobulins was also depleted of its inhibitory activity; (c) inhibition of adhesion to primary nonkeratinized oral mucosa cells; (d) dose-dependent inhibition of Strep. pneumoniae adhesion to HaCat cells by bacterial cell wall (CW) and membrane (M) proteins (□ BSA control; CW protein concentration; ▪ M protein concentration). These experiments were repeated on 3 separate experiments in triplicate each time, and a representative experiment is illustrated. cfu stands for cfu/well. The results are the mean of the triplicate wells ± standard error of the mean (s.e.m.).
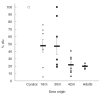
Fig. 5
Development of inhibitory activity against Strep. pneumoniae adhesion to epithelial cells in children compared to adult. Sera were obtained longitudinally at 18, 30 and 42 months (m) of age (n = 8) from healthy children attending day care centres, with documented Strep. pneumoniae carriage. Individual adult’s sera (n = 5) were obtained from randomly chosen healthy adults. In a representative experiment HaCat cells were incubated with 106 Strep. pneumoniae cfu in the absence (control) or presence of these sera diluted 1:250. The number of bacteria bound to the cells was determined as decribed in the Methods section. Individual results are presented as % adhered bacteria. Marked are the calculated means and the standard errors of mean (s.e.m.).
Similar articles
- Human antibodies to PhtD, PcpA, and Ply reduce adherence to human lung epithelial cells and murine nasopharyngeal colonization by Streptococcus pneumoniae.
Kaur R, Surendran N, Ochs M, Pichichero ME. Kaur R, et al. Infect Immun. 2014 Dec;82(12):5069-75. doi: 10.1128/IAI.02124-14. Epub 2014 Sep 22. Infect Immun. 2014. PMID: 25245804 Free PMC article. - Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse.
Ling E, Feldman G, Portnoi M, Dagan R, Overweg K, Mulholland F, Chalifa-Caspi V, Wells J, Mizrachi-Nebenzahl Y. Ling E, et al. Clin Exp Immunol. 2004 Nov;138(2):290-8. doi: 10.1111/j.1365-2249.2004.02628.x. Clin Exp Immunol. 2004. PMID: 15498039 Free PMC article. - Identification of a human immunodominant B-cell epitope within the immunoglobulin A1 protease of Streptococcus pneumoniae.
De Paolis F, Beghetto E, Spadoni A, Montagnani F, Felici F, Oggioni MR, Gargano N. De Paolis F, et al. BMC Microbiol. 2007 Dec 18;7:113. doi: 10.1186/1471-2180-7-113. BMC Microbiol. 2007. PMID: 18088426 Free PMC article. - Synchrony in serum antibody response to conserved proteins of Streptococcus pneumoniae in young children.
Ren D, Almudevar AL, Pichichero ME. Ren D, et al. Hum Vaccin Immunother. 2015;11(2):489-97. doi: 10.4161/21645515.2014.990861. Hum Vaccin Immunother. 2015. PMID: 25692218 Free PMC article. - Correlates of immunity to Group A Streptococcus: a pathway to vaccine development.
Frost H, Excler JL, Sriskandan S, Fulurija A. Frost H, et al. NPJ Vaccines. 2023 Jan 17;8(1):1. doi: 10.1038/s41541-022-00593-8. NPJ Vaccines. 2023. PMID: 36650164 Free PMC article. Review.
Cited by
- Streptococcus pneumoniae Cell-Wall-Localized Phosphoenolpyruvate Protein Phosphotransferase Can Function as an Adhesin: Identification of Its Host Target Molecules and Evaluation of Its Potential as a Vaccine.
Mizrachi Nebenzahl Y, Blau K, Kushnir T, Shagan M, Portnoi M, Cohen A, Azriel S, Malka I, Adawi A, Kafka D, Dotan S, Guterman G, Troib S, Fishilevich T, Gershoni JM, Braiman A, Mitchell AM, Mitchell TJ, Porat N, Goliand I, Chalifa Caspi V, Swiatlo E, Tal M, Ellis R, Elia N, Dagan R. Mizrachi Nebenzahl Y, et al. PLoS One. 2016 Mar 18;11(3):e0150320. doi: 10.1371/journal.pone.0150320. eCollection 2016. PLoS One. 2016. PMID: 26990554 Free PMC article. - NADH oxidase functions as an adhesin in Streptococcus pneumoniae and elicits a protective immune response in mice.
Muchnik L, Adawi A, Ohayon A, Dotan S, Malka I, Azriel S, Shagan M, Portnoi M, Kafka D, Nahmani H, Porgador A, Gershoni JM, Morrison DA, Mitchell A, Tal M, Ellis R, Dagan R, Nebenzahl YM. Muchnik L, et al. PLoS One. 2013;8(4):e61128. doi: 10.1371/journal.pone.0061128. Epub 2013 Apr 8. PLoS One. 2013. PMID: 23577197 Free PMC article. - Vaccine-induced human antibodies to PspA augment complement C3 deposition on Streptococcus pneumoniae.
Ochs MM, Bartlett W, Briles DE, Hicks B, Jurkuvenas A, Lau P, Ren B, Millar A. Ochs MM, et al. Microb Pathog. 2008 Mar;44(3):204-14. doi: 10.1016/j.micpath.2007.09.007. Epub 2007 Oct 11. Microb Pathog. 2008. PMID: 18006268 Free PMC article. - Pneumococcal 6-phosphogluconate-dehydrogenase, a putative adhesin, induces protective immune response in mice.
Daniely D, Portnoi M, Shagan M, Porgador A, Givon-Lavi N, Ling E, Dagan R, Mizrachi Nebenzahl Y. Daniely D, et al. Clin Exp Immunol. 2006 May;144(2):254-63. doi: 10.1111/j.1365-2249.2006.03047.x. Clin Exp Immunol. 2006. PMID: 16634799 Free PMC article. - Immunoproteomics: the key to discovery of new vaccine antigens against bacterial respiratory infections.
Dennehy R, McClean S. Dennehy R, et al. Curr Protein Pept Sci. 2012 Dec;13(8):807-15. doi: 10.2174/138920312804871184. Curr Protein Pept Sci. 2012. PMID: 23305366 Free PMC article. Review.
References
- Centre for Disease Control and Prevention. Pneumonia and influenza death rate – United States 1979–94. Morbid. Mortal. Weekly Report. 1995;44:535–7. - PubMed
- Austrian R. The enduring pneumococcus: Unfinished business and opportunities for the future. In: Tomasz A, editor. Mary Ann Liebert Inc. Streptococcus pneumoniae Larchmont; 1995. pp. 3–6. - PubMed
- Robbins JB, Austrian R, Lee CJ, et al. Considerations for formulating the second-generation of pneumococal capsular polysaccharide vaccine with emphasis on the cross-reactive type within groups. J Infect Dis. 1983;148:1136–59. - PubMed
- Cowan MJ, Ammann AJ, Wara DW, Howie VM, Schultz L, Doyle N, Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978;62:721–7. - PubMed
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical