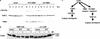SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint - PubMed (original) (raw)
SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint
Parvin T Yazdi et al. Genes Dev. 2002.
Abstract
Structural maintenance of chromosomes (SMC) proteins (SMC1, SMC3) are evolutionarily conserved chromosomal proteins that are components of the cohesin complex, necessary for sister chromatid cohesion. These proteins may also function in DNA repair. Here we report that SMC1 is a component of the DNA damage response network that functions as an effector in the ATM/NBS1-dependent S-phase checkpoint pathway. SMC1 associates with BRCA1 and is phosphorylated in response to IR in an ATM- and NBS1-dependent manner. Using mass spectrometry, we established that ATM phosphorylates S957 and S966 of SMC1 in vivo. Phosphorylation of S957 and/or S966 of SMC1 is required for activation of the S-phase checkpoint in response to IR. We also discovered that the phosphorylation of NBS1 by ATM is required for the phosphorylation of SMC1, establishing the role of NBS1 as an adaptor in the ATM/NBS1/SMC1 pathway. The ATM/CHK2/CDC25A pathway is also involved in the S-phase checkpoint activation, but this pathway is intact in NBS cells. Our results indicate that the ATM/NBS1/SMC1 pathway is a separate branch of the S-phase checkpoint pathway, distinct from the ATM/CHK2/CDC25A branch. Therefore, this work establishes the ATM/NBS1/SMC1 branch, and provides a molecular basis for the S-phase checkpoint defect in NBS cells.
Figures
Figure 1
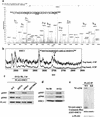
SMC1 associates with BRCA1 and is phosphorylated in response to IR. (a) Coimmunoprecipitation of BRCA1 and SMC1 in HeLa NE and NE treated with 1.2 μg/mL ethidium bromide. SGT1 is an irrelevant antibody serving as a negative control. (b) Phosphorylation of SMC1 in response to IR. T24 cells were left untreated, irradiated with 10 Gy of IR and incubated for 2 h, or treated with 1 mM hydroxyurea (HU) for 8 h. Cell lysates before and after treatment with 20 U/μL λ protein phosphatase were analyzed by Western blotting.
Figure 2
Phosphorylation of SMC1 in response to IR is ATM- and NBS1-dependent. (a) Comparison of SMC1 phosphorylation kinetics in normal (GM00637) and A-T (GM05849) cell lines. Cells were irradiated with 10 Gy and incubated for indicated time periods. Cell lysates were analyzed by Western blotting. (b) Comparison of SMC1 phosphorylation in normal and A-T cells as a function of IR dosage. After receiving the indicated IR dosages, cells were incubated for 1 h. (c,d) Comparison of SMC1 phosphorylation in normal (IMR-90 and TK6) and NBS1-defective cells (GM07166 and JS). (e) Dependence of SMC1 phosphorylation on ATM. ATM fibroblasts FT169A and their ATM cDNA-complemented derivative cell line YZ5 were irradiated and analyzed for SMC1 phosphorylation. (f) Dependence of SMC1 and BRCA1 phosphorylation on NBS1. A GM07166 TERT cell line and its NBS1 cDNA-complemented derivative cell line were irradiated and analyzed for SMC1 and BRCA1 phosphorylation.
Figure 3
S957 and S966 of SMC1 are phosphorylated in vivo in response to IR. SMC1 was immunoprecipitated from HeLa NE prepared from cells that were irradiated with 20 Gy of IR, recovered for 2 h, and resolved on a 6% SDS-PAGE gel. The top and bottom bands of SMC1 were cut out and analyzed separately. Identification of phosphopeptides was carried out as described using mass spectrometry (Zhang et al. 1998). (a) The MS/MS spectrum of the tryptic phosphopeptide amino acids 945–968 of SMC1 from the bottom band. The spectrum identifies the site of phosphorylation as S957. (b) A portion of the MALDI-TOF spectra of the Asp-N digests of the top band of SMC1 that were treated with (bottom panel) and without (top panel) calf intestine phosphatase (CIP). The arrow marks the phosphopeptide, in which S966 is tentatively assigned as the site of phosphorylation. (c) Test of the specificity of phospho-specific antibodies. Flag-SMC1 WT, S957A, S966A, and S957A/S966A were expressed in 293T cells. Cells were treated with 10 Gy of IR and allowed to recover for 1 h. Flag-SMC1 proteins were immunoprecipitated with a Flag antibody, and Western blotted with phospho-specific antibodies against pS957 and pS966. (d) In vivo phosphorylation of S957 and S966 of SMC1 in response to IR. Untreated and IR-treated 293T cells were analyzed by Western blotting using phospho-specific antibodies against pS957 and pS966. (e) In vitro phosphorylation of SMC1 by ATM. A GST–SMC1 fragment (amino acids 890–1233) and Flag-tagged ATM (wild-type or kinase dead) immunoprecipitated by Flag antibody from transiently transfected 293T cells following 20 Gy of IR and 1 h of recovery were used to perform the kinase assay.
Figure 4
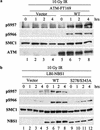
Phosphorylation of S957 and S966 of SMC1 depends on ATM and NBS1. (a) Vector and wild-type ATM cDNA-complemented A-T cells were irradiated with 10 Gy of IR and allowed to recover for the indicated times. Phosphorylation of S957 and S966 of SMC1 was examined by Western blotting using phospho-specific antibodies against pS957 and pS966 of SMC1. Two identical gels were run, one for blotting pS957, and the other for blotting pS966. Care was taken to load the two gels equally. (b) Vector, wild-type NBS1, and phosphorylation mutant S278A/S343A NBS1 cDNA-complemented NBS cells were irradiated with 10 Gy of IR and allowed to recover for the indicated times. Phosphorylations of S957 and S966 of SMC1 were examined by Western blotting using phospho-specific antibodies against pS957 and pS966 of SMC1.
Figure 5
Phosphorylation of S957 and S966 of SMC1 does not depend on BRCA1 or BLM. (a) Vector and wild-type BRCA1 cDNA-complemented HCC1937 cells were irradiated with 10 Gy of IR and allowed to recover for the indicated times. Phosphorylation of S957 and S966 of SMC1 was examined by Western blotting using phospho-specific antibodies against pS957 and pS966 of SMC1. (b) Phosphorylation of SMC1 was examined by SDS-PAGE mobility shift in the HCC1937 cells that were complemented with vector and wild-type BRCA1 cDNA. (c) The BLM-deficient cell line GM03402 was used to examine the kinetics of SMC1 phosphorylation in response to 10 Gy of IR.
Figure 6
Phosphorylation of S957 and/or S966 of SMC1 is required for IR-induced S-phase checkpoint activation. RDS in 293T cells that were transiently transfected with pCDNA3-Flag vector, pCNDA3-Flag–SMC1-WT, S957A, S966A, S957A/S966A, and pCDNA3-Flag–ATM-KD. Incorporated [3H]thymidine was measured following 20 Gy of IR and 1 h of incubation. Data were normalized with respect to untreated (control) cells. Error bars represent the standard deviation. Five independent experiments were carried out.
Figure 7
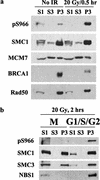
Characterization of SMC1 phosphorylation. (a) Phosphorylation of S966 does not affect SMC1 chromatin binding. U2OS cells were fractionated into the cytoplasmic (S1), nucleoplasmic (S2), and chromatin (P3) fractions and analyzed by Western blotting. (b) Cohesion per se is not sufficient for S966 phosphorylation. U2OS cells were blocked in mitosis with nocodazole, irradiated with 20 Gy of IR, and allowed to recover for 2 h. Cells were then separated into the mitotic fraction (M) and G1/S/G2 fraction by mitotic shake-off. M and G1/S/G2 cells were subject to chromatin fractionation.
Figure 8
The ATM/NBS1/SMC1 branch of the S-phase checkpoint. (a) Kinetics of degradation and accumulation of CDC25A in response to 10 Gy of IR in NBS cells that were complemented with the vector, wild-type, and S278A/S343A NBS1 cDNA. (b) CHK2 activation in response to 10 Gy of IR and 1 h of recovery in NBS cells complemented with the vector, wild-type, and S278A/S343A NBS1 cDNA and A-T cells complemented with the vector and wild-type ATM cDNA. CHK2 was detected by Western blotting. The slowly migrating form marked with an arrow is labeled with Pi-CHK2. (c) A simplified S-phase checkpoint pathway in response to DSBs in mammalian cells. Phosphorylated NBS1 (Pi-NBS1) is depicted as an adaptor.
Similar articles
- Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage.
Kim ST, Xu B, Kastan MB. Kim ST, et al. Genes Dev. 2002 Mar 1;16(5):560-70. doi: 10.1101/gad.970602. Genes Dev. 2002. PMID: 11877376 Free PMC article. - Distinct functions of Nijmegen breakage syndrome in ataxia telangiectasia mutated-dependent responses to DNA damage.
Lee JH, Xu B, Lee CH, Ahn JY, Song MS, Lee H, Canman CE, Lee JS, Kastan MB, Lim DS. Lee JH, et al. Mol Cancer Res. 2003 Jul;1(9):674-81. Mol Cancer Res. 2003. PMID: 12861053 - Requirement for NBS1 in the S phase checkpoint response to DNA methylation combined with PARP inhibition.
Horton JK, Stefanick DF, Zeng JY, Carrozza MJ, Wilson SH. Horton JK, et al. DNA Repair (Amst). 2011 Feb 7;10(2):225-34. doi: 10.1016/j.dnarep.2010.11.003. Epub 2010 Dec 3. DNA Repair (Amst). 2011. PMID: 21130714 Free PMC article. - Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis.
Taylor AM, Groom A, Byrd PJ. Taylor AM, et al. DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1219-25. doi: 10.1016/j.dnarep.2004.04.009. DNA Repair (Amst). 2004. PMID: 15279810 Review. - The ATM-dependent DNA damage signaling pathway.
Kitagawa R, Kastan MB. Kitagawa R, et al. Cold Spring Harb Symp Quant Biol. 2005;70:99-109. doi: 10.1101/sqb.2005.70.002. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869743 Review.
Cited by
- SMRT compounds abrogate cellular phenotypes of ataxia telangiectasia in neural derivatives of patient-specific hiPSCs.
Lee P, Martin NT, Nakamura K, Azghadi S, Amiri M, Ben-David U, Perlman S, Gatti RA, Hu H, Lowry WE. Lee P, et al. Nat Commun. 2013;4:1824. doi: 10.1038/ncomms2824. Nat Commun. 2013. PMID: 23652012 - Recent advances in the nucleolar responses to DNA double-strand breaks.
Korsholm LM, Gál Z, Nieto B, Quevedo O, Boukoura S, Lund CC, Larsen DH. Korsholm LM, et al. Nucleic Acids Res. 2020 Sep 25;48(17):9449-9461. doi: 10.1093/nar/gkaa713. Nucleic Acids Res. 2020. PMID: 32857853 Free PMC article. Review. - miR-638 suppresses DNA damage repair by targeting SMC1A expression in terminally differentiated cells.
He M, Lin Y, Tang Y, Liu Y, Zhou W, Li C, Sun G, Guo M. He M, et al. Aging (Albany NY). 2016 Jul;8(7):1442-56. doi: 10.18632/aging.100998. Aging (Albany NY). 2016. PMID: 27405111 Free PMC article. - ATM promotes apoptosis and suppresses tumorigenesis in response to Myc.
Pusapati RV, Rounbehler RJ, Hong S, Powers JT, Yan M, Kiguchi K, McArthur MJ, Wong PK, Johnson DG. Pusapati RV, et al. Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1446-51. doi: 10.1073/pnas.0507367103. Epub 2006 Jan 23. Proc Natl Acad Sci U S A. 2006. PMID: 16432227 Free PMC article. - Nse1, Nse2, and a novel subunit of the Smc5-Smc6 complex, Nse3, play a crucial role in meiosis.
Pebernard S, McDonald WH, Pavlova Y, Yates JR 3rd, Boddy MN. Pebernard S, et al. Mol Biol Cell. 2004 Nov;15(11):4866-76. doi: 10.1091/mbc.e04-05-0436. Epub 2004 Aug 25. Mol Biol Cell. 2004. PMID: 15331764 Free PMC article.
References
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. - PubMed
- Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous