Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis - PubMed (original) (raw)
Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis
Sean D Conner et al. J Cell Biol. 2002.
Abstract
The mu 2 subunit of the AP2 complex is known to be phosphorylated in vitro by a copurifying kinase, and it has been demonstrated recently that mu 2 phosphorylation is required for transferrin endocytosis (Olusanya, O., P.D. Andrews, J.R. Swedlow, and E. Smythe. 2001. Curr. Biol. 11:896-900). However, the identity of the endogenous kinase responsible for this phosphorylation is unknown. Here we identify and characterize a novel member of the Prk/Ark family of serine/threonine kinases, adaptor-associated kinase (AAK)1. We find that AAK1 copurifies with adaptor protein (AP)2 and that it directly binds the ear domain of alpha-adaptin in vivo and in vitro. In neuronal cells, AAK1 is enriched at presynaptic terminals, whereas in nonneuronal cells it colocalizes with clathrin and AP2 in clathrin-coated pits and at the leading edge of migrating cells. AAK1 specifically phosphorylates the mu subunit in vitro, and stage-specific assays for endocytosis show that mu phosphorylation by AAK1 results in a decrease in AP2-stimulated transferrin internalization. Together, these results provide strong evidence that AAK1 is the endogenous mu 2 kinase and plays a regulatory role in clathrin-mediated endocytosis. These results also lend support to the idea that clathrin-mediated endocytosis is controlled by cycles of phosphorylation/desphosphorylation.
Figures
Figure 1.
The predicted amino acid sequence of the AAK1. The S/T kinase domain is underlined, whereas the COOH-terminal domain, which was identified by phage display, is in bold. This COOH-terminal domain was also used to generate polyclonal antibodies. The clathrin, EH domain, and α-adaptin interaction motifs, DLL, NPF, and DPF, respectively, are boxed.
Figure 2.
AAK1 shares strong identity to the Ark family of S/T kinases in the kinase domain. Sequence data available from GenBank/EMBL/DDBJ under accession nos.: AAK1, NP_055726; DKFZp434p0116, NP_060063; Numb-associated kinase (NAK), AF15596; human cycling-associated kinase (hGAK), NP_005246; rat cycling-associated kinase (rGAK), NP_112292; Prk1p, NP_012171; Ark1p, NP_014378. Top bar indicates amino acid number.
Figure 3.
AAK1 interacts directly with α-adaptin in vitro. AAK1–GST fusion proteins, COOH-terminal domain, or full-length AAK1 consisting of amino acids 679–893 and 1–893, respectively, immobilized on glutathione-agarose beads were incubated with the COOH-terminal domain of α-adaptin (amino acids 701–938, lane 2) or rat brain cytosol (RBC, lanes 1 and 5). Immunoblot analysis probing for the presence of α-adaptin reveals that AAK1 is capable of interacting directly with the COOH-terminal domain of α-adaptin (lane 2) and the full-length protein in rat brain cytosol (lanes 1 and 5). This interaction is likely through AAK1 DPF motifs. pAb 0927 and mAb AP.6 were used to detect full-length α-adaptin and its COOH-terminal domain, respectively.
Figure 4.
AAK1 is enriched in bovine brain membrane fractions and associated with isolated CCV and AP2 preparations. (A) Immunoblot analysis reveals that AAK1 (visualized with pAb 5366) cofractionates with both clathrin (visualized with mAb TD.1) and APs (visualized with mAb 100-1 that recognizes both β1 and β2 subunits), which are found in the low (P1) and high (P2) speed membrane pellets of homogenized bovine brain. Treatment of the high speed membrane pellet, which is highly enriched for clathrin-coated vesicles, with 0.75 M Tris, pH 7, extracts the majority of AAK1 to a soluble pool (ES); however, a fraction of AAK1 remains membrane associated when the extracted membranes are repelleted (EP). Each lane represents equivalent fractions of each sample. (B) AAK1 is found associated with isolated APs (lane 1), clathrin (lane 2), and CCVs (lane 3), and CCVs from rat liver (lane 4). Cr, crude bovine brain homogenate; S1, low speed supernatant; S2, high speed supernatant (cytosol).
Figure 5.
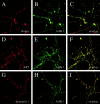
Immunolocalization of AAK1 in rat hippocampal neurons. Rat hippocampal neurons were incubated with rhodamine-dextran (A), which marks sites of endocytosis, and then fixed and stained with pAb 5366 to detect AAK1 (B). AAK1 colocalizes with sites active in endocytosis (C, yellow overlay). Likewise, AAK1 immunolabel (E and H) is found to colocalize with regions that are highly enriched in AP2 (D, mAb AP.6 immunolabel) and dynamin 1 (G, mAb hudy1 immunolabel) as revealed by their overlays (F and I). Images were visualized by confocal microscopy.
Figure 6.
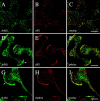
AAK1 associates with endocytic structures in HeLa cells. AAK1 (A) shows good colocalization with AP2 (B, mAb AP.6 immunolabel) in nonmigrating HeLa cells as observed in their overlay (C, yellow). In migrating HeLa cells, AAK1 is highly enriched at the leading edge (D and G, arrow) where it colocalizes with AP2 (E) and clathrin (H, mAb X22 immunolabel). Immunolocalizations visualized by confocal microscopy. Bars, 5 μm.
Figure 7.
AAK1 preferentially phosphorylates the μ subunit. (A) Isolated baculovirus-expressed AAK1–GST fusion protein is capable of autophosphorylation in vitro when incubated with [γ-32P]ATP (lane 1). Likewise, incubation of AAK1–GST with fractions enriched in APs shows an increase in phosphorylation of a ∼50-kD protein over APs alone (arrowhead, lane 2 compared with 3). (B) The ∼50-kD band, which is preferentially phosphorylated by AAK1, was identified as μ by immunoprecipitation with pAbs against μ1 and μ2.
Figure 8.
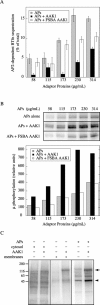
Phosphorylation of μ by AAK1 inhibits AP2-stimulated BTfn sequestration. (A) APs stimulate the sequestration of BTfn in a concentration-dependent manner in stage-specific in vitro endocytosis assays that measure AP2-stimulated constricted coated pit formation (grey bars). However, the addition of 30 μg/ml recombinant AAK1–GST fusion protein results in a decrease in AP2-dependent stimulation (black bars). In contrast, addition of 30 μg/ml FSBA-treated AAK1–GST fusion protein has little effect on AP2 stimulation up to an AP concentration of 173 μg/ml (white bars). Results are the mean ± SEM of six experiments. (B) AAK1-dependent inhibition directly correlates with the phosphorylation of μ. The autoradiogram shows that increased μ phosphorylation is observed when increasing concentrations of APs are incubated with [γ-32P]ATP. Likewise, the addition of 30 μg/ml of AAK1–GST fusion protein results in a significant increase in μ phosphorylation, whereas 30 μg/ml FSBA-treated AAK1–GST fusion protein shows only endogenous levels of μ phosphorylation. Note that we sometimes resolve a μ doublet on gradient gels in our kinase assays. Phosphorylated μ was quantitated (graph below) using the ImageQuant Software package from Molecular Dynamics, and the graph represents the mean of two kinase experiments. (C) AAK1 specifically phosphorylates the μ subunit of APs. APs, K562 cytosol, and A431 membrane preparations were each incubated with [γ-32P]ATP in the presence or absence of AAK1–GST. Only the μ subunit (arrowhead) of APs is significantly phosphorylated as a result of additional AAK1–GST (AAK1 autophosphorylation, arrow).
Similar articles
- Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals.
Ricotta D, Conner SD, Schmid SL, von Figura K, Honing S. Ricotta D, et al. J Cell Biol. 2002 Mar 4;156(5):791-5. doi: 10.1083/jcb.200111068. Epub 2002 Mar 4. J Cell Biol. 2002. PMID: 11877457 Free PMC article. - Differential requirements for AP-2 in clathrin-mediated endocytosis.
Conner SD, Schmid SL. Conner SD, et al. J Cell Biol. 2003 Sep 1;162(5):773-9. doi: 10.1083/jcb.200304069. J Cell Biol. 2003. PMID: 12952931 Free PMC article. - AAK1-mediated micro2 phosphorylation is stimulated by assembled clathrin.
Conner SD, Schröter T, Schmid SL. Conner SD, et al. Traffic. 2003 Dec;4(12):885-90. doi: 10.1046/j.1398-9219.2003.0142.x. Traffic. 2003. PMID: 14617351 - Clathrin coat construction in endocytosis.
Pearse BM, Smith CJ, Owen DJ. Pearse BM, et al. Curr Opin Struct Biol. 2000 Apr;10(2):220-8. doi: 10.1016/s0959-440x(00)00071-3. Curr Opin Struct Biol. 2000. PMID: 10753805 Review. - Clathrin adaptors really adapt.
Kirchhausen T. Kirchhausen T. Cell. 2002 May 17;109(4):413-6. doi: 10.1016/s0092-8674(02)00751-1. Cell. 2002. PMID: 12086597 Review.
Cited by
- Dopamine D1A directly interacts with otoferlin synaptic pathway proteins: Ca2+ and phosphorylation underlie an NSF-to-AP2mu1 molecular switch.
Selvakumar D, Drescher MJ, Deckard NA, Ramakrishnan NA, Morley BJ, Drescher DG. Selvakumar D, et al. Biochem J. 2017 Jan 1;474(1):79-104. doi: 10.1042/BCJ20160690. Epub 2016 Nov 7. Biochem J. 2017. PMID: 27821621 Free PMC article. - Temporal phosphoproteomic analysis of VEGF-A signaling in HUVECs: an insight into early signaling events associated with angiogenesis.
Abhinand CS, Galipon J, Mori M, Ramesh P, Prasad TSK, Raju R, Sudhakaran PR, Tomita M. Abhinand CS, et al. J Cell Commun Signal. 2023 Sep;17(3):1067-1079. doi: 10.1007/s12079-023-00736-z. Epub 2023 Mar 7. J Cell Commun Signal. 2023. PMID: 36881336 Free PMC article. - Phosphoserine acidic cluster motifs bind distinct basic regions on the μ subunits of clathrin adaptor protein complexes.
Singh R, Stoneham C, Lim C, Jia X, Guenaga J, Wyatt R, Wertheim JO, Xiong Y, Guatelli J. Singh R, et al. J Biol Chem. 2018 Oct 5;293(40):15678-15690. doi: 10.1074/jbc.RA118.003080. Epub 2018 Aug 22. J Biol Chem. 2018. PMID: 30135209 Free PMC article. - JC Polyomavirus Entry by Clathrin-Mediated Endocytosis Is Driven by β-Arrestin.
Mayberry CL, Soucy AN, Lajoie CR, DuShane JK, Maginnis MS. Mayberry CL, et al. J Virol. 2019 Apr 3;93(8):e01948-18. doi: 10.1128/JVI.01948-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700597 Free PMC article. - AP2M1 mediates autophagy-induced CLDN2 (claudin 2) degradation through endocytosis and interaction with LC3 and reduces intestinal epithelial tight junction permeability.
Ganapathy AS, Saha K, Suchanec E, Singh V, Verma A, Yochum G, Koltun W, Nighot M, Ma T, Nighot P. Ganapathy AS, et al. Autophagy. 2022 Sep;18(9):2086-2103. doi: 10.1080/15548627.2021.2016233. Epub 2021 Dec 29. Autophagy. 2022. PMID: 34964704 Free PMC article.
References
- Bar-Zvi, D., and D. Branton. 1986. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin beta-light chain by casein kinase II. J. Biol. Chem. 261:9614–9621. - PubMed
- Bauerfeind, R., K. Takei, and P. De Camilli. 1997. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 272:30984–30992. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous