Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells - PubMed (original) (raw)
Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells
Heiko Runz et al. J Neurosci. 2002.
Abstract
Generation of amyloid-beta (Abeta) from the amyloid precursor protein (APP) requires proteolytic cleavage by two proteases, beta- and gamma-secretase. Several lines of evidence suggest a role for cholesterol on secretase activities, although the responsible cellular mechanisms remain unclear. Here we show that alterations in cholesterol transport from late endocytic organelles to the endoplasmic reticulum have important consequences for both APP processing and the localization of gamma-secretase-associated presenilins (PS). Exposure of neuronal cells to cholesterol transport-inhibiting agents resulted in a marked decrease in beta-cleavage of full-length APP. In contrast, gamma-secretase activity on APP C-terminal fragments was enhanced, increasing the production of both Abeta40 and Abeta42. Remarkably, retention of cholesterol in endosomal/lysosomal compartments induced PS1 and PS2 to accumulate in Rab7-positive vesicular organelles implicated in cholesterol sorting. Accumulation of PS in vesicular compartments was prominent in both Chinese hamster ovary cells deficient in Niemann-Pick C1 protein as well as in neuronal cells exposed to the cholesterol transport-inhibiting agent U18666A. Because Abeta42 also localized to PS1-containing vesicular compartments, organelles involved in cholesterol transport might represent an important site for gamma-secretase activity. Our results suggest that the subcellular distribution of cholesterol may be an important factor in how cholesterol alters Abeta production and the risk of Alzheimer's disease.
Figures
Fig. 1.
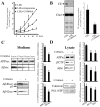
Class 2 amphiphiles inhibit intracellular cholesterol transport and decrease β-cleavage of APP in neuronal cells. SH-SY5Y cells (A) or mature rat cortical neurons in culture (B) were labeled with14C-cholesterol in LDL for 2–12 hr (A) or 24 hr (B).14C-cholesterol ester formation in cells incubated in the presence or absence of 3 μg/ml (for neuroblastoma cells) or 0.75 μg/ml (for primary neurons) U18666A or 80 μ
m
imipramine was monitored by the extraction of lipids and subsequent TLC. Depicted are densitometric quantifications of 14C-cholesterol ester signal intensities as a percentage of the total 14C activity per lane (means from three experiments). CE, Cholesterol ester; Chol, cholesterol. C, SFV-infected mature rat cortical neurons were incubated for 8 hr with LDL at different concentrations of U18666A. Conditioned medium was immunoprecipitated with W02, followed by Western blot detection with W02. Immunoprecipitations of conditioned media from neurons exposed to LDL and either 0.75 μg/ml U18666A (+) or not (−) with antibodies G2-10 or G2-11 were detected with G2-10 to visualize secretory Aβ40 and p3 or with W02 for the detection of secretory Aβ42, respectively.D, Western blot of respective neuronal lysates with W02 (after W02 immunoprecipitation), anti-calnexin antibody, or antibody 95.23 against PS1 NTF. C, D, Quantifications of intracellular (ic) and secreted (sec) APP, βCTF, and overall Aβ levels (n = 4–7 experiments). Depicted are ratios of signal intensities from U18666A- or imipramine-treated versus untreated primary cortical neurons or SH-SY5Y cells. Error bars indicate 1 SD.
Fig. 2.
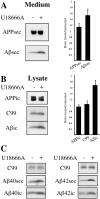
U18666A increases intracellular and secretory Aβ levels in SP-C99-transfected SH-SY5Y cells. Human SH-SY5Y neuroblastoma cells stably transfected with APP C-terminal fragment SP-C99 were incubated for 24 hr with 50 μg/ml LDL in the presence (+) or absence (−) of 3 μg/ml U18666A. Conditioned medium (A) and cell lysates (B) were immunoprecipitated with antibody W02, followed by Western blot detection with W02. Graphs show quantification of intracellular (ic) and secreted (sec) endogenous APP, C99, and overall Aβ levels (n = 5 experiments). Depicted are ratios of signal intensities from U18666A-treated versus untreated cells. Error bars indicate 1 SD. C, Medium and lysates of SH-SY5Y cells were immunoprecipitated with antibodies G2-10 and G2-11 specific for Aβ40 and Aβ42, respectively, and were detected with W02. Western blots with W02 show effects of U18666A treatment on secretory and intracellular Aβ species compared with C99.
Fig. 3.
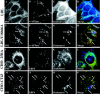
Inhibiting intracellular cholesterol transport induces an accumulation of PS1 in vesicular compartments associated with late endosomes and lysosomes. Human SH-SY5Y neuroblastoma cells were incubated for 24 hr with 50 μg/ml LDL in the presence (E–H) or absence (A–D) of 3 μg/ml U18666A. After fixation in paraformaldehyde the cells were triple labeled with polyclonal PS1 antibody 95.23 (A, E;green fluorescence), monoclonal antibody Osw2 to v-ATPase as a late endosomal/lysosomal marker (B, F;red fluorescence), and filipin for the detection of cellular cholesterol (C, G; _blue_fluorescence). Parental CHO-25RA cells (with regular sterol distribution) and CHO-CT43 cells (expressing a nonfunctional NPC1 protein) were incubated with 50 μg/ml LDL for 24 hr, fixed with paraformaldehyde, and triple labeled with PS1 antibody 95.23 (I, M), filipin (K, O), and the monoclonal antibody 6C4 against LBPA to visualize late endosomes (J, N; red fluorescence). D, H, L, P, Merged images. Arrows indicate selected regions positive for all three markers. Scale bars, 10 μm.
Fig. 4.
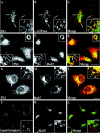
PS-containing compartments are positive for Rab7 and surround late endosomes in a ring-like manner. SH-SY5Y (A–C) or A431 cells (D–L) were incubated for 24 hr with 50 μg/ml LDL and 3 μg/ml U18666A. After fixation in paraformaldehyde the SH-SY5Y cells were stained against PS1 (A, C; green) and v-ATPase (B, C; red). Fixed A431 cells stably overexpressing Rab7-GFP (E, H, K;green) were labeled with a monoclonal PS1 antibody (D, F; red), polyclonal PS2 antibody (G, I; red), or Oil red O to visualize neutral lipids (J, L; red). C, F, I, L, Merged images. Scale bars, 8 μm. Arrows_indicate selected vesicles positive for both markers.Insets show single sections from confocal stacks of random vesicular structures in cell bodies. Scale bars in_insets, 1 μm.
Fig. 5.
Rab7-containing compartments after U18666A exposure of hippocampal neurons are distinct from cholesterol-retaining late endosomes and retain Aβ42 and PS1. Mature hippocampal neurons were incubated with LDL and U18666A for 24 hr, fixed, and stained with filipin (B, E;red) and either monoclonal antibody 6C4 against LBPA (A, C; green) or a polyclonal antibody against Rab7 (D, F; green). G, H, Coimmunostainings of 95.23 for PS1 and monoclonal antibody G2-11 to detect cellular Aβ42. A–C and_insets_ in D–I show areas in the cell body of a hippocampal neuron. C, Merged image from_A, B_. F, Merged image from D, E. I, Merged image from G, H. Arrows indicate selected compartments. Scale bars, 4 μm.
Fig. 6.
PS1-bearing vesicular compartments in hippocampal neurons impaired in cholesterol transport exclude β′COP and BiP but retain calnexin. Fully polarized rat hippocampal neurons in culture were incubated with 50 μg/ml LDL and 0.75 μg/ml U18666A for 24 hr. Neurons were fixed and triple labeled with antibody 95.23 (A, E, I), filipin (C, G, K), and monoclonal antibodies against β′COP (B), BiP/GRP78 (F), or calnexin (J). D, H, L, Merged images. Bottom insets show selected regions of single sections in the cell body of the depicted neuron (top insets). Arrows indicate selected compartments. Scale bars, 3 μm.
Fig. 7.
U18666A decreases cholesterol ester formation independently of PS1 expression. Cultured mouse fibroblasts from wild-type (PS1+/+PS2+/+), PS1 single knock-out (PS1−/−PS2+/+), and PS1/PS2 double knock-out (PS1−/−PS2−/−) mice were labeled with14C-cholesterol in LDL in the presence (+) or absence (−) of 3 μg/ml U18666A for 24 hr. Lipids were extracted and separated by TLC. Effects of U18666A on CE formation in treated cells at equal CE levels in untreated cells are shown.
Similar articles
- Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi.
Xia W, Zhang J, Ostaszewski BL, Kimberly WT, Seubert P, Koo EH, Shen J, Selkoe DJ. Xia W, et al. Biochemistry. 1998 Nov 24;37(47):16465-71. doi: 10.1021/bi9816195. Biochemistry. 1998. PMID: 9843412 - The discrepancy between presenilin subcellular localization and gamma-secretase processing of amyloid precursor protein.
Cupers P, Bentahir M, Craessaerts K, Orlans I, Vanderstichele H, Saftig P, De Strooper B, Annaert W. Cupers P, et al. J Cell Biol. 2001 Aug 20;154(4):731-40. doi: 10.1083/jcb.200104045. Epub 2001 Aug 13. J Cell Biol. 2001. PMID: 11502763 Free PMC article. - Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.
Steiner H, Capell A, Leimer U, Haass C. Steiner H, et al. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review. - Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.
Xia W. Xia W. Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
Cited by
- Alterations in gene expression in mutant amyloid precursor protein transgenic mice lacking Niemann-Pick type C1 protein.
Maulik M, Thinakaran G, Kar S. Maulik M, et al. PLoS One. 2013;8(1):e54605. doi: 10.1371/journal.pone.0054605. Epub 2013 Jan 29. PLoS One. 2013. PMID: 23382922 Free PMC article. - Neurodegeneration in Niemann-Pick Type C Disease: An Updated Review on Pharmacological and Non-Pharmacological Approaches to Counteract Brain and Cognitive Impairment.
Cariati I, Masuelli L, Bei R, Tancredi V, Frank C, D'Arcangelo G. Cariati I, et al. Int J Mol Sci. 2021 Jun 20;22(12):6600. doi: 10.3390/ijms22126600. Int J Mol Sci. 2021. PMID: 34202978 Free PMC article. Review. - Amyloid precursor protein controls cholesterol turnover needed for neuronal activity.
Pierrot N, Tyteca D, D'auria L, Dewachter I, Gailly P, Hendrickx A, Tasiaux B, Haylani LE, Muls N, N'kuli F, Laquerrière A, Demoulin JB, Campion D, Brion JP, Courtoy PJ, Kienlen-Campard P, Octave JN. Pierrot N, et al. EMBO Mol Med. 2013 Apr;5(4):608-25. doi: 10.1002/emmm.201202215. EMBO Mol Med. 2013. PMID: 23554170 Free PMC article. - Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes.
Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Vetrivel KS, et al. J Biol Chem. 2004 Oct 22;279(43):44945-54. doi: 10.1074/jbc.M407986200. Epub 2004 Aug 17. J Biol Chem. 2004. PMID: 15322084 Free PMC article. - Impacts of membrane biophysics in Alzheimer's disease: from amyloid precursor protein processing to aβ Peptide-induced membrane changes.
Askarova S, Yang X, Lee JC. Askarova S, et al. Int J Alzheimers Dis. 2011 Mar 17;2011:134971. doi: 10.4061/2011/134971. Int J Alzheimers Dis. 2011. PMID: 21547213 Free PMC article.
References
- Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, St. George-Hyslop P, Cordell B, Fraser P, De Strooper B. Presenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J Cell Biol. 1999;147:277–294. - PMC - PubMed
- Armogida M, Petit A, Vincent B, Scarzello S, Alves da Costa C, Checler F. Endogenous β-amyloid production in presenilin-deficient embryonic mouse fibroblasts. Nat Cell Biol. 2001;3:1030–1033. - PubMed
- Bouillot C, Prochiantz A, Rougon G, Allinquant B. Axonal amyloid precursor protein expressed by neurons in vitro is present in a membrane fraction with caveolae-like properties. J Biol Chem. 1996;271:7640–7644. - PubMed
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ. Niemann–Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. - PubMed
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Periak-Vance MA. Gene dose of apolipoprotein type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical