Genetic dysmyelination alters the molecular architecture of the nodal region - PubMed (original) (raw)
Genetic dysmyelination alters the molecular architecture of the nodal region
Edgardo J Arroyo et al. J Neurosci. 2002.
Abstract
We have examined the molecular organization of axons in the spinal cords of myelin-deficient (md) rats, which have profound CNS dysmyelination associated with oligodendrocyte cell death. Although myelin sheaths are rare, most large axons are at least partially surrounded by oligodendrocyte processes. At postnatal day 7 (P7), almost all node-like clusters of voltage-gated Na+ channels and ankyrinG are adjacent to axonal segments ensheathed by oligodendrocytes, but at P21, many node-like clusters are found in axonal segments that lack oligodendrocyte ensheathment. In P21 wild-type (WT) rats, the voltage-gated Na+ channels Na(v)1.2, Na(v)1.6, and Na(v)1.8, are found in different subpopulations of myelinated axons, and md rats have a similar distribution. The known molecular components of paranodes--contactin, Caspr, and neurofascin 155--are not clustered in md spinal cords, and no septate-like junctions between oligodendrocyte processes and axons are found by electron microscopy. Furthermore, Kv1.1 and Kv1.2 K+ channels are not spatially segregated from the node-like clusters of Na+ channels in md rats, in contrast to their WT littermates. These results suggest the following: node-like clusters of voltage-gated Na+ channels and ankyrinG form adjacent to ensheathed axonal segments even in the absence of a myelin sheath; these clusters persist after oligodendrocyte cell death; dysmyelination does not alter the expression of different nodal of voltage-gated Na+ channels; the absence of paranodes results in the mislocalization of neurofascin155, contactin, and Caspr, and the aberrant localization of Kv1.1 and Kv1.2.
Figures
Fig. 1.
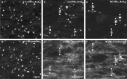
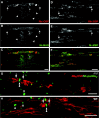
Node-like clusters of voltage-gated Na+ channels in md spinal cord. These images were taken from longitudinal sections of the ventral funiculus from P21 md (A–D) or WT (E, F) spinal cords, immunostained with a rabbit antiserum against ankyrinG (A, C, E; TRITC) and a monoclonal antibody against voltage-gated Na+channels (B; FITC) or tenascin-R (D, F; FITC). Note that node-like clusters of colocalize with voltage-gated Na+ channels and tenascin-R. The_pairs_ of arrowheads mark the some of the node-like clusters in C–F. Scale bars, 10 μm.
Fig. 2.
Similar localization of voltage-gated Na+ channels in P21 md and WT spinal cords. These are images from transverse sections of the cervical spinal cord of P21 md and WT rats, immunostained for Nav1.2, Nav1.6, and Nav1.8. The midline is indicated by pairs of double arrowheads. There is diffuse Nav1.2 staining in the corticospinal tract (cst) and the adjacent gray matter of the dorsal horn (dh); Nav1.8 staining is mainly found in the membrane of neuronal cell bodies (arrowheads); Nav1.6 staining is found in the majority of nodes and initial segments (arrows). A few myelinated fibers in the dorsal columns (dc) have Nav1.2 and Nav1.8 staining. The overall distribution of these Na+ channels is not altered in_md_ rats. Scale bar, 50 μm.
Fig. 3.
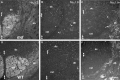
Nav1.6 is the predominant voltage-gated Na+ channel in P21 _md_and WT spinal cords; Shaker_-type K+channels are not separated from Na+ channels in_md spinal cord. A–F are taken from transverse sections of the ventral funiculus from an _md_rat (A–C) and its WT littermate (D–F), double-labeled with a pan-Na+ channel monoclonal antibody (A, D; fluorescein) and a rabbit antiserum against Nav1.6 (B, E; rhodamine); _C_and F show the merged images. Note the crescent-shaped node-like clusters in md spinal cords, and that some node-like clusters are Nav1.6–negative (arrows). G and H are taken from a longitudinal section of an md(G) or a WT (H) spinal cord, double labeled with a rabbit antiserum against Kv1.2 (TRITC) and a pan-Na+ channel monoclonal antibody (FITC). In the md spinal cord, Kv1.2 abuts or even overlaps (arrows) with node-like clusters of Na+ channels, whereas the unstained paranodal region separates the two types of channels in WT spinal cords. Scale bars, 10 μm.
Fig. 4.
Immunoblot analysis of voltage-gated Na+ channels, contactin, and Caspr. Homogenates of spinal cords and sciatic nerves were prepared from P21_md_ and their WT littermates, and 100 μg of protein was analyzed for Nav1.2, Nav1.6, Nav1.8, Caspr, and contactin. For Nav1.2, Nav1.6, Nav1.8, the films were exposed for 20 min, then rehybridized with a mouse monoclonal antibody to GAPDH, and exposed to film for 5 min. For Caspr, the film was exposed for 30 sec, then rehybridized with a mouse monoclonal antibody to GAPDH, and exposed to film for 30 sec. For contactin, the film was exposed for 5 sec, then rehybridized with a mouse monoclonal antibody to GAPDH, and exposed to film for 2 min. The Nav1.2, Nav1.6, and Nav1.8, bands were all ∼250 kDa; the Caspr doublet band ∼190 kDa; the contactin band ∼135 kDa. Note the similar amounts of Nav1.2, Nav1.6, Nav1.8, Caspr, and contactin in md and WT samples.
Fig. 5.
Caspr and neurofascin are not localized to CNS paranodes in md rats. These images were made from longitudinal sections of P21 md(A–C) or WT (D–F) spinal cord, after double-labeling with a rabbit antiserum against NF155 (A, D; TRITC) and a mouse monoclonal antibody against Caspr (B, E; FITC); C and_F_ show the merged images. In md rats, Caspr and neurofascin are colocalized in the paranodes in the ventral roots (arrows) but not in the spinal cord. The_arrowhead_ marks an incisure, which is stained for NF155 but not for Caspr (Tait et al., 2000). In WT rats, Caspr and NF155 are colocalized at all CNS paranodes. Asterisks mark oligodendrocyte cell bodies, which are stained for NF155 but not Caspr (Tait et al., 2000); double arrowheads mark nodes. Scale bar, 10 μm.
Fig. 6.
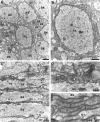
Electron microscopy of P21 md_spinal cord. These are electron micrographs of transverse (A, B) and longitudinal (C–E) sections of the ventromedial funiculus. In A and C, note several large axons (ax) that are not myelinated.A shows a portion of an oligodendrocyte (ol) with dilated cisternae; these are common in_md rats. B shows one axon in higher magnification; note the multiple processes (asterisks) surrounding the axon. D and E show the rectangular regions; note the astrocytic processes (as) in D and the stack of five oligodendrocyte processes in_E_.
Fig. 7.
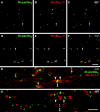
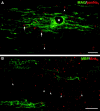
MAG-, MBP-, and OSP-positive oligodendrocytes ensheathe axons in md spinal cord. These images are taken from longitudinal sections of a P21 md(A–G) or a WT (H) spinal cord, double labeled with a rabbit antiserum against OSP (TRITC) and a monoclonal antibodies (FITC) against MAG (A–C), MBP (C–F), or pan-Na+ channels (G, H). As in WT rats (G), the paranodes in md_rats contain a spiral of OSP staining (arrows), and (MBP- and MAG-positive) internodes–oligodendrocyte processes often contain OSP-positive strands. An asterisk marks an oligodendrocyte nucleus in A–C. In G and_H, paranodal OSP staining (arrows) flanks most node-like clusters of Na+ channels (double arrowheads) in WT rats, but many node-like clusters are not associated with paranodal OSP staining in_md_ rats. Scale bars: A–C, G, H, 10 μm; D–F, 20 μm.
Fig. 8.
Clusters of Na+ channels and ankryinG in regions devoid of oligodendrocytes. These images were taken from longitudinal sections of P21 _md_spinal cord, stained with a rabbit antiserum against MAG and a pan-Na+ channel monoclonal antibody (A; merged confocal images), or a rabbit antiserum against ankyrinG and a mouse monoclonal antibody against MBP (B; merged epifluorescence images). Note the clusters of Na+ channels and ankyrinGstaining in regions that are devoid of MAG–MBP staining (arrowheads), as well as adjacent to MAG–MBP-positive processes (arrows). An asterisk marks an oligodendrocyte nucleus. Scale bars: A, 10 μm;B, 20 μm.
Fig. 9.
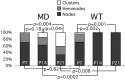
Quantitative analysis of node-like clusters in the ventral funiculus. Longitudinal sections through the ventral funiculus of P7 (4 md and 4 WT), P14 (2_md_ and 2 WT), and P21 (2 md and 2 WT) were double-labeled with the pan Na+ channel monoclonal antibody (to label nodes) and the rabbit antiserum against MAG (to label ensheathed axonal segments). All node-like clusters of Na+ channels were classified as either naked clusters (not flanked by MAG-positive ensheathed axonal segments), heminodes (flanked on only side one by MAG-positive axonal segments), or nodes (flanked on both sides by MAG-positive axonal segments). The percentage of nodes was calculated at each age; ANOVA statistical analyses were used to compare the samples; the p values are shown for each comparison.
Fig. 10.
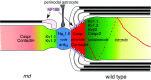
The organization of the axonal membrane in_md_ rats. In this schematic image, the axon is depicted as intact, whereas the glial cells are depicted as being hemisected, to reveal the axoglial junctions. The localization of nodal (blue; voltage-gated Na+ channels and ankyrinG), paranodal (red for contactin and Caspr; purple for NF155), and juxtaparanodal proteins (green; Kv1.1, Kv1.2, Kvβ2, Caspr2) in WT rats are shown on the right. The_left side_ of the figure depicts that in P21_md_ rats, nodal proteins can be localized with or without oligodendrocyte ensheathment, whereas contactin and Caspr are diffusely localized, and Kv1.1 and Kv1.2 abut the nodal membrane.
Similar articles
- Kv3.1b is a novel component of CNS nodes.
Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. Devaux J, et al. J Neurosci. 2003 Jun 1;23(11):4509-18. doi: 10.1523/JNEUROSCI.23-11-04509.2003. J Neurosci. 2003. PMID: 12805291 Free PMC article. - Molecular organization of the nodal region is not altered in spontaneously diabetic BB-Wistar rats.
Brown AA, Xu T, Arroyo EJ, Levinson SR, Brophy PJ, Peles E, Scherer SS. Brown AA, et al. J Neurosci Res. 2001 Jul 15;65(2):139-49. doi: 10.1002/jnr.1137. J Neurosci Res. 2001. PMID: 11438983 - K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve.
Rasband MN, Trimmer JS, Peles E, Levinson SR, Shrager P. Rasband MN, et al. J Neurocytol. 1999 Apr-May;28(4-5):319-31. doi: 10.1023/a:1007057512576. J Neurocytol. 1999. PMID: 10739574 - Molecular organization and function of vertebrate septate-like junctions.
Faivre-Sarrailh C. Faivre-Sarrailh C. Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183211. doi: 10.1016/j.bbamem.2020.183211. Epub 2020 Feb 4. Biochim Biophys Acta Biomembr. 2020. PMID: 32032590 Review. - The local differentiation of myelinated axons at nodes of Ranvier.
Poliak S, Peles E. Poliak S, et al. Nat Rev Neurosci. 2003 Dec;4(12):968-80. doi: 10.1038/nrn1253. Nat Rev Neurosci. 2003. PMID: 14682359 Review.
Cited by
- Caspr regulates the processing of contactin and inhibits its binding to neurofascin.
Gollan L, Salomon D, Salzer JL, Peles E. Gollan L, et al. J Cell Biol. 2003 Dec 22;163(6):1213-8. doi: 10.1083/jcb.200309147. Epub 2003 Dec 15. J Cell Biol. 2003. PMID: 14676309 Free PMC article. - KCNQ2 is a nodal K+ channel.
Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. Devaux JJ, et al. J Neurosci. 2004 Feb 4;24(5):1236-44. doi: 10.1523/JNEUROSCI.4512-03.2004. J Neurosci. 2004. PMID: 14762142 Free PMC article. - Initiation of sodium channel clustering at the node of Ranvier in the mouse optic nerve.
Ishibashi T, Ikenaka K, Shimizu T, Kagawa T, Baba H. Ishibashi T, et al. Neurochem Res. 2003 Jan;28(1):117-25. doi: 10.1023/a:1021608514646. Neurochem Res. 2003. PMID: 12587670 - No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse.
Lasiene J, Shupe L, Perlmutter S, Horner P. Lasiene J, et al. J Neurosci. 2008 Apr 9;28(15):3887-96. doi: 10.1523/JNEUROSCI.4756-07.2008. J Neurosci. 2008. PMID: 18400887 Free PMC article. - Mutation in the myelin proteolipid protein gene alters BK and SK channel function in the caudal medulla.
Mayer CA, Macklin WB, Avishai N, Balan K, Wilson CG, Miller MJ. Mayer CA, et al. Respir Physiol Neurobiol. 2009 Dec 31;169(3):303-14. doi: 10.1016/j.resp.2009.09.013. Epub 2009 Oct 4. Respir Physiol Neurobiol. 2009. PMID: 19808102 Free PMC article.
References
- Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibers. Histochem Cell Biol. 2000;113:1–18. - PubMed
- Arroyo EJ, Xu T, Poliak S, Watson M, Peles E, Scherer SS. Internodal specializations of myelinated axons in the CNS. Cell Tissue Res. 2001;305:53–66. - PubMed
- Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K+ channel clustering. J Neurosci Res. 1999;58:752–764. - PubMed
- Baron P, Kamholz J, Scherer SS, Honda Scherer, Shy M, Scarpini E, Scarlato G, Pleasure D. Appearance of PLP mRNA in specific regions of the developing rat lumbosacral spinal cord as revealed by in situ hybridization. Exp Neurol. 1993;121:139–147. - PubMed
- Barron KD, Dentinger MP, Csiza CK, Keegan SM, Mankes R. Abnormalities of central axons in a dysmyelinative rat mutant. Exp Mol Pathol. 1987;47:125–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS08075/NS/NINDS NIH HHS/United States
- NS36637/NS/NINDS NIH HHS/United States
- NS34528/NS/NINDS NIH HHS/United States
- G0000221/MRC_/Medical Research Council/United Kingdom
- NS37100/NS/NINDS NIH HHS/United States
- R01 NS036637/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources