Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors - PubMed (original) (raw)
Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors
Raewyn M Seaberg et al. J Neurosci. 2002.
Abstract
Neurogenesis persists in two adult brain regions: the ventricular subependyma and the subgranular cell layer in the hippocampal dentate gyrus (DG). Previous work in many laboratories has shown explicitly that multipotential, self-renewing stem cells in the subependyma are the source of newly generated migrating neurons that traverse the rostral migratory stream and incorporate into the olfactory bulb as interneurons. These stem cells have been specifically isolated from the subependyma, and their properties of self-renewal and multipotentiality have been demonstrated in vitro. In contrast, it is a widely held assumption that the "hippocampal" stem cells that can be isolated in vitro from adult hippocampus reside in the neurogenic subgranular layer and represent the source of new granule cell neurons, but this has never been tested directly. Primary cell isolates derived from the precise microdissection of adult rodent neurogenic regions were compared using two very different commonly used culture methods: a clonal colony-forming (neurosphere) assay and a monolayer culture system. Importantly, both of these culture methods generated the same conclusion: stem cells can be isolated from hippocampus-adjacent regions of subependyma, but the adult DG proper does not contain a population of resident neural stem cells. Indeed, although the lateral ventricle and other ventricular subependymal regions directly adjacent to the hippocampus contain neural stem cells that exhibit long-term self-renewal and multipotentiality, separate neuronal and glial progenitors with limited self-renewal capacity are present in the adult DG, suggesting that neuron-specific progenitors and not multipotential stem cells are the source of newly generated DG neurons throughout adulthood.
Figures
Fig. 1.
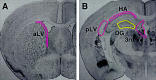
Dissection and rate of sphere formation of adult mouse neurogenic regions. A, B, Atlas images of adult mouse brain sections (Franklin and Paxinos, 1997) adapted to show the microdissection of viable 500 μm vibratome sections used to isolate tissue from neurogenic regions.A, Coronal section through the anterior lateral ventricle (aLV) with the dissected region highlighted. Note that this dissection includes both subependymal and ependymal tissue, but for the purposes of this study ependymal sphere formation was ignored. B, Coronal section through the hippocampus. Note that the dentate gyrus (DG) dissection excludes all regions containing subependymal tissue; these regions were dissected and cultured separately. 3rd V, Third ventricle; pLV, posterior lateral ventricle;HA, hippocampal arch. This dissection scheme was used for both rats and mice.
Fig. 2.
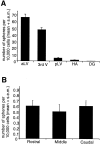
A, Comparison of the numbers of primary spheres generated from different neurogenic regions in the adult mouse brain. The data are expressed as the mean (+SEM) number of spheres generated per 10,000 cells plated (i.e., per well; density = 20 cells per microliter). Tissue from each region (aLV, anterior lateral ventricle; pLV, posterior lateral ventricle; 3rd V, third ventricle;HV, hippocampal arch; DG, dentate gyrus) was dissociated into single cells and plated in serum-free media containing EGF + FGF2 and B27 supplement; spheres were counted at 7 DIV. Note that the aLV cells generate neurospheres at a rate ∼120-fold higher than the DG cells (0.54 ± 0.1), on the basis of data from n > 120 animals and 10 separate experiments. B, Comparison of the number of spheres generated from adult dentate gyrus at different rostrocaudal levels. Rostral, middle, and caudal sections through the DG yield a similar number of sphere colonies. Tissue from each individual 500 μm section (n = 85 sections from >20 animals) through the DG was separately dissociated into single cells and cultured at 20 cells per microliter; spheres were counted at 7 DIV.
Fig. 3.
A, B, Comparison of the self-renewal ability of adult anterior lateral ventricle (subependymal) and dentate gyrus spheres. A, The data are expressed as the mean (+SEM) number of spheres generated per single sphere dissociation. At each passage, individual spheres (n > 60 spheres per condition) were dissociated per tissue culture well, and the number of sphere colonies that formed was counted after 7 DIV. Although the adult aLV neurosphere-initiating cells demonstrated self-renewal by giving rise to secondary and tertiary neurospheres (as did the adult pLV, 3rd V, and HA neurospheres; data not shown), the adult DG sphere-initiating cells did not demonstrate self-renewal and did not give rise to secondary sphere colonies. Individual spheres were dissociated and replated in identical media conditions as were used for primary culture (EGF + FGF2 with B27 supplement). B, The data are expressed as the percentage of individual spheres that passaged to generate new spheres. Procedures were followed as described for A. Note that nearly 100% of adult aLV spheres give rise to new spheres at each passage.
Fig. 4.
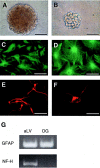
A, B, Comparison of the size of primary spheres derived from adult anterior lateral ventricle (subependyma) (A) and dentate gyrus (B). Note that the DG spheres are smaller than the aLV spheres. Scale bars, 100 μm.C_–_G, Comparison of the ability of adult aLV- and DG-derived primary spheres to contribute to different neural cell lineages. Primary adult aLV neurospheres generate both GFAP+ astrocytes (C) and βIII-tubulin+ neurons (E), whereas adult DG spheres generate only GFAP+astrocytes (D). Small adult DG clumps generate only βIII-tubulin+ neurons (F). Note that although both cell types can be derived from adult aLV or DG cultures, the aLV-derived neurons and glia are generated by a common precursor, whereas the DG-derived neurons and glia are derived from separate progenitors. Individual spheres or clumps were plated on MATRIGEL basement membrane matrix in 1% FBS for 7–8 DIV and then processed for immunocytochemistry. Scale bars, 50 μm. G, Neuronal and glial gene expression were confirmed using RT-PCR. RNA was isolated from differentiated aLV and DG sphere colonies. Primers were used to detect GFAP (150 bp) and NF-H (452 bp). Sphere colonies derived from subependymal tissue (aLV) generate both neuronal (NF-H) and glial (GFAP) progeny, whereas colonies derived from dentate gyrus cells (DG) generate differentiated progeny that express GFAP but do not express NF-H. Data are representative of at least three separate experiments.
Fig. 5.
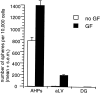
Comparison of the sphere-forming ability of primary tissue from adult rat neurogenic regions and a clonal cell line of adult hippocampal progenitors in the presence and absence of growth factors (GF). The data are expressed as the mean (+SEM) number of spheres generated per 10,000 cells plated (i.e., per well; density = 20 cells per microliter). Cells were plated in either serum-free media (no GF) or serum-free media with EGF and FGF2 (GF). AHPs were compared with primary adult rat tissue (DG, dentate gyrus;aLV, anterior lateral ventricle). Resultant spheres were counted at 7 DIV. Note that both AHPs and primary aLV spheres (12 ± 2) were generated in no GF, whereas zero spheres arose from adult DG in the absence of growth factors (0.0 ± 0.0). A small number of DG spheres were generated in GF(1.5 ± 0.3).
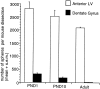
Fig. 6.
Comparison of the numbers of spheres formed from PND1, PND10, and adult anterior lateral ventricle (subependyma) and dentate gyrus. The data are expressed as the mean (+SEM) number of spheres generated per brain dissection. Tissue was dissected from adult, PND1, and PND10 brains (n > 40 animals from 5 separate experiments) from viable vibratome sections and cultured in EGF + FGF2 with B27 supplement at 20 cells per microliter. Spheres were counted at 7 DIV. Cells from the PND1 DG generated 30-fold more spheres than adult DG cells, whereas cells from the PND1, PND10, and adult aLV did not generate significantly different numbers of spheres.
Similar articles
- Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus.
Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Hattiangady B, et al. Stem Cells. 2007 Aug;25(8):2104-17. doi: 10.1634/stemcells.2006-0726. Epub 2007 May 17. Stem Cells. 2007. PMID: 17510219 - Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics.
Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Chiasson BJ, et al. J Neurosci. 1999 Jun 1;19(11):4462-71. doi: 10.1523/JNEUROSCI.19-11-04462.1999. J Neurosci. 1999. PMID: 10341247 Free PMC article. - Origins, functions, and potential of adult neural stem cells.
Kuhn HG, Svendsen CN. Kuhn HG, et al. Bioessays. 1999 Aug;21(8):625-30. doi: 10.1002/(SICI)1521-1878(199908)21:8<625::AID-BIES1>3.0.CO;2-6. Bioessays. 1999. PMID: 10440858 Review. - [Adult neurogenesis in physiological and pathological conditions].
Kaneko N, Sawamoto K. Kaneko N, et al. Brain Nerve. 2008 Apr;60(4):319-28. Brain Nerve. 2008. PMID: 18421973 Review. Japanese.
Cited by
- The neurosphere assay: an effective in vitro technique to study neural stem cells.
Soares R, Ribeiro FF, Lourenço DM, Rodrigues RS, Moreira JB, Sebastião AM, Morais VA, Xapelli S. Soares R, et al. Neural Regen Res. 2021 Nov;16(11):2229-2231. doi: 10.4103/1673-5374.310678. Neural Regen Res. 2021. PMID: 33818505 Free PMC article. No abstract available. - Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia.
Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, Pinteaux E. Rodriguez-Grande B, et al. J Neuroinflammation. 2015 Jan 24;12:15. doi: 10.1186/s12974-014-0227-y. J Neuroinflammation. 2015. PMID: 25616391 Free PMC article. - Embryonic cortical neural stem cells migrate ventrally and persist as postnatal striatal stem cells.
Willaime-Morawek S, Seaberg RM, Batista C, Labbé E, Attisano L, Gorski JA, Jones KR, Kam A, Morshead CM, van der Kooy D. Willaime-Morawek S, et al. J Cell Biol. 2006 Oct 9;175(1):159-68. doi: 10.1083/jcb.200604123. J Cell Biol. 2006. PMID: 17030986 Free PMC article. - Mammalian neural stem-cell renewal: nature versus nurture.
Arsenijevic Y. Arsenijevic Y. Mol Neurobiol. 2003 Feb;27(1):73-98. doi: 10.1385/MN:27:1:73. Mol Neurobiol. 2003. PMID: 12668902 Review. - Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation.
Nakano I, Paucar AA, Bajpai R, Dougherty JD, Zewail A, Kelly TK, Kim KJ, Ou J, Groszer M, Imura T, Freije WA, Nelson SF, Sofroniew MV, Wu H, Liu X, Terskikh AV, Geschwind DH, Kornblum HI. Nakano I, et al. J Cell Biol. 2005 Aug 1;170(3):413-27. doi: 10.1083/jcb.200412115. J Cell Biol. 2005. PMID: 16061694 Free PMC article.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–336. - PubMed
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. - PubMed
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. - PubMed
- Cameron HA, McKay RDG. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. - PubMed
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical