Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes - PubMed (original) (raw)
Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes
Georg Kochs et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Bunyaviruses replicate in the cytoplasm of infected cells. New viral particles are formed by budding of nucleocapsids into the Golgi apparatus. We have previously shown that the IFN-induced human MxA protein inhibits bunyavirus replication by an unknown mechanism. Here we demonstrate that MxA binds to the nucleocapsid protein of La Crosse virus (LACV) and colocalizes with the viral protein in cytoplasmic complexes. Electron microscopy revealed that these complexes accumulated in the perinuclear area and consisted of highly ordered fibrillary structures. A similar MxA-mediated redistribution of viral nucleocapsid proteins was detected with other bunyaviruses, such as Bunyamwera virus and Rift Valley fever virus. MxA(E645R), a carboxy-terminal mutant of MxA without antiviral activity against LACV, did not lead to complex formation. Wild-type MxA, but not MxA(E645R), was able to bind to LACV nucleocapsid protein in coimmunoprecipitation assays, demonstrating the importance of the carboxy-terminal effector domain of MxA. These results illustrate an efficient mechanism of IFN action whereby an essential virus component is trapped in cytoplasmic inclusions and becomes unavailable for the generation of new virus particles.
Figures
Figure 1
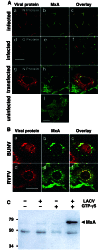
MxA binds to the N protein of bunyaviruses and colocalizes with N in large perinuclear complexes. (A) MxA-expressing Vero cells were either infected with 10 pfu of LACV per cell (a_–_f) or were transfected with plasmid pSC-N coding for the N protein of LACV (g–i, note that the transfected cell is surrounded by nontransfected cells expressing only MxA) or were left untreated (j). Cells were fixed 24 h later and analyzed by immunofluorescence for the presence of viral N or G protein (red) and MxA (green), using specific Abs. (B) MxA-expressing Vero cells were infected with 20 pfu per cell of either BUNV or RVFV for 20 h, fixed, and analyzed for MxA and N expression. The cells were stained with Abs directed against the N proteins of either BUNV (red; a) or RVFV (d) together with an MxA-specific Ab (green; b and e). Panels at the right show the superimposition of the two images. Bars, 20 μm. (C) MxA-expressing Vero cells were infected with 10 pfu of LACV per cell for 20 h or were left untreated. Cell lysates were used for immunoprecipitation in the presence or absence of GTPγS by using the N-specific antiserum. The coprecipitated proteins were detected by Western-blotting with the MxA-specific mAb M143. Positions of _M_r markers (in kDa) are indicated at the left.
Figure 2
Ultrastructure of MxA/N complexes. MxA-expressing Vero cells (a) or control cells (b) were infected with 10 pfu of LACV per cell for 20 h. Cells were prepared for transmission EM by cellulose capillary technique. The nucleus is marked by Nu. For immuno-EM, MxA-expressing cells infected with LACV were prepared by using the progressive lowering of temperature method. Thin sections of the cells were labeled with the N-specific Ab (c) or with mAb M143 directed against MxA (d). Primary Abs were detected with gold-labeled protein A. The magnification is indicated by the bars. The diameter of the gold particles is 10 nm.
Figure 3
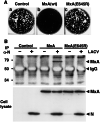
MxA(E645R) lacks antiviral activity against LACV and does not interact with the viral N protein. (A) Plaque formation of LACV is not inhibited by MxA(E645R). Vero cells expressing wild-type MxA, MxA(E645R), or control cells were infected with 200 pfu of LACV and stained with crystal violet 5 days later. (B) MxA(E645R) does not coimmunoprecipitate with N of LACV. Vero cells expressing MxA or MxA(E645R) and control cells were infected with 10 pfu per cell of LACV or were left untreated. Cell lysates were prepared 20 h later and immunoprecipitated with the N-specific antiserum in the presence of GTPγS. The immunoprecipitate was analyzed for the presence of MxA with mAb M143 by immunoblot (Upper). The coprecipitated background bands visible in all lanes are of unknown origin. The expression levels of MxA, MxA(E645R), and N were assessed by immunoblot analysis of the crude cell lysates, using the same Abs (Lower).
Figure 4
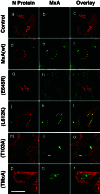
The antiviral activity of MxA correlates with the formation of MxA/N complexes. Control cells (a_–_c) or Vero cells constitutively expressing MxA (d–f) or MxA(E645R) (g–i) were used for infection. In parallel, Vero cells that transiently expressed MxA(L612K) (j–l), MxA(T103A) (m–o), or TMxA (p–r) were used. All cells were infected with 10 pfu/cell of LACV. Twenty hours later, the cells were fixed and analyzed for the viral N (red) and the various MxA proteins (green) by immunofluorescence. Panels at the right show the superimposition of the two images. Bar, 20 μm.
Similar articles
- Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity.
Dick A, Graf L, Olal D, von der Malsburg A, Gao S, Kochs G, Daumke O. Dick A, et al. J Biol Chem. 2015 May 15;290(20):12779-92. doi: 10.1074/jbc.M115.650325. Epub 2015 Mar 31. J Biol Chem. 2015. PMID: 25829498 Free PMC article. - Human MxA protein inhibits the replication of Crimean-Congo hemorrhagic fever virus.
Andersson I, Bladh L, Mousavi-Jazi M, Magnusson KE, Lundkvist A, Haller O, Mirazimi A. Andersson I, et al. J Virol. 2004 Apr;78(8):4323-9. doi: 10.1128/jvi.78.8.4323-4329.2004. J Virol. 2004. PMID: 15047845 Free PMC article. - Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein.
Frese M, Kochs G, Feldmann H, Hertkorn C, Haller O. Frese M, et al. J Virol. 1996 Feb;70(2):915-23. doi: 10.1128/JVI.70.2.915-923.1996. J Virol. 1996. PMID: 8551631 Free PMC article. - Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity.
Haller O, Kochs G. Haller O, et al. Traffic. 2002 Oct;3(10):710-7. doi: 10.1034/j.1600-0854.2002.31003.x. Traffic. 2002. PMID: 12230469 Review. - Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity.
Haller O, Kochs G. Haller O, et al. J Interferon Cytokine Res. 2011 Jan;31(1):79-87. doi: 10.1089/jir.2010.0076. Epub 2010 Dec 19. J Interferon Cytokine Res. 2011. PMID: 21166595 Review.
Cited by
- Bunyaviruses and the type I interferon system.
Elliott RM, Weber F. Elliott RM, et al. Viruses. 2009 Dec;1(3):1003-21. doi: 10.3390/v1031003. Epub 2009 Nov 23. Viruses. 2009. PMID: 21994579 Free PMC article. - Mx proteins: antiviral gatekeepers that restrain the uninvited.
Verhelst J, Hulpiau P, Saelens X. Verhelst J, et al. Microbiol Mol Biol Rev. 2013 Dec;77(4):551-66. doi: 10.1128/MMBR.00024-13. Microbiol Mol Biol Rev. 2013. PMID: 24296571 Free PMC article. Review. - Evolution and Antiviral Specificities of Interferon-Induced Mx Proteins of Bats against Ebola, Influenza, and Other RNA Viruses.
Fuchs J, Hölzer M, Schilling M, Patzina C, Schoen A, Hoenen T, Zimmer G, Marz M, Weber F, Müller MA, Kochs G. Fuchs J, et al. J Virol. 2017 Jul 12;91(15):e00361-17. doi: 10.1128/JVI.00361-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28490593 Free PMC article. - Rapid Reversible Osmoregulation of Cytoplasmic Biomolecular Condensates of Human Interferon-α-Induced Antiviral MxA GTPase.
Sehgal PB, Yuan H, Jin Y. Sehgal PB, et al. Int J Mol Sci. 2022 Oct 22;23(21):12739. doi: 10.3390/ijms232112739. Int J Mol Sci. 2022. PMID: 36361529 Free PMC article. - Differential antiviral response of endothelial cells after infection with pathogenic and nonpathogenic hantaviruses.
Kraus AA, Raftery MJ, Giese T, Ulrich R, Zawatzky R, Hippenstiel S, Suttorp N, Krüger DH, Schönrich G. Kraus AA, et al. J Virol. 2004 Jun;78(12):6143-50. doi: 10.1128/JVI.78.12.6143-6150.2004. J Virol. 2004. PMID: 15163707 Free PMC article.
References
- Haller O, Frese M, Kochs G. Rev Sci Tech OIE. 1998;17:220–230. - PubMed
- Staeheli P, Pitossi F, Pavlovic J. Trends Cell Biol. 1993;3:268–272. - PubMed
- Sever S, Damke H, Schmid S L. Traffic. 2000;1:385–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases