Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) - PubMed (original) (raw)
Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac)
Per Svenningsson et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Fluoxetine (Prozac) is the most widely prescribed medication for the treatment of depression. Nevertheless, little is known about the molecular basis of its clinical efficacy, apart from the fact that fluoxetine increases the synaptic availability of serotonin. Here we show that, in vivo, fluoxetine, given either acutely or chronically, regulates the phosphorylation state of dopamine- and cAMP-regulated phosphoprotein of M(r) 32,000 (DARPP-32) at multiple sites in prefrontal cortex, hippocampus, and striatum. Acute administration of fluoxetine increases phosphorylation of DARPP-32 at the protein kinase A site, Thr-34, and at the casein kinase-1 site, Ser-137, and decreases phosphorylation at the cyclin-dependent kinase 5 site, Thr-75. Each of these changes contributes, through distinct signaling pathways, to increased inhibition of protein phosphatase-1, a major serine/threonine protein phosphatase in the brain. Fluoxetine also increases phosphorylation of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluR1 at Ser-831 and Ser-845. Both the fluoxetine-mediated increase in AMPA receptor phosphorylation at Ser-845-GluR1 and the beneficial responsiveness to fluoxetine in an animal test of antidepressant efficacy were strongly reduced in DARPP-32 knockout mice, indicating a critical role for this phosphoprotein in the antidepressant actions of fluoxetine. Mice chronically treated with fluoxetine had increased levels of DARPP-32 mRNA and protein and a decreased ability to increase phospho-Ser-137-DARPP-32 and phospho-Ser-831-GluR1. These chronic changes may be relevant to the delayed onset of therapeutic efficacy of fluoxetine.
Figures
Figure 1
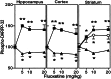
Regulation of DARPP-32 phosphorylation in vivo by acute treatment with fluoxetine. Data are shown for mice treated with saline or fluoxetine (5, 10, or 20 mg/kg) and killed 15-min postinjection. The amounts of (■) phospho-Thr-34–DARPP-32 and (▴) phospho-Thr-75–DARPP-32 were quantified in hippocampus, prefrontal cortex, and striatum, and the amounts of (○) phospho-Ser-137–DARPP-32 were quantified in striatum. Data represent means ± SE for six to ten mice per group. *,P < 0.05, **, P< 0.01 compared with saline-treated mice, one-way ANOVA followed by Dunnett's test.
Figure 2
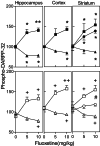
Regulation of DARPP-32 phosphorylation in vivo by chronic treatment with fluoxetine. Data are shown for mice treated for 19 days with saline (Upper) or fluoxetine (10 mg/kg;Lower) and then challenged with fluoxetine (0, 5, or 10 mg/kg in saline) and killed 15-min postinjection. The amounts of (■, □) phospho-Thr-34–, (▴, ▵) phospho-Thr-75– and (●, ○) phospho-Ser-137–DARPP-32 were quantified and normalized to total DARPP-32 (see text). Data represent means ± SE for four to eight mice per group. *, P < 0.05, **, P < 0.01 compared with saline/saline-treated mice; +, P < 0.05, ++,P < 0.01 compared with fluoxetine/saline-treated mice; #, P < 0.05 compared with saline/fluoxetine-treated mice, one-way ANOVA followed by Dunnett's test.
Figure 3
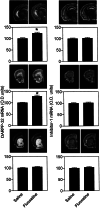
Levels of DARPP-32 mRNA and inhibitor-1 after chronic treatment for 19 days with saline or fluoxetine (10 mg/kg). The exposure time for the dark-field photomicrographs was 7 days, or, in the case of striatal DARPP-32 mRNA, 2 days. The amounts of DARPP-32 mRNA (Left) and inhibitor-1 mRNA (Right) were quantified by densitometry in hippocampus (Top), prefrontal cortex (Middle), and striatum (Bottom). Data represent means ± SE for four to six mice per group. *, P < 0.05 compared with saline-treated mice, Student's t test.
Figure 4
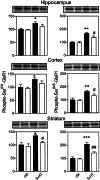
Regulation of DARPP-32 phosphorylation by serotonin (100 μM)in vitro. The amounts of phospho-Thr-34–DARPP-32 (Upper) and phospho-Thr-75–DARPP-32 (Lower) in extracts of slices were quantified by densitometry. Data represent means ± SE for three to ten experiments. *, P < 0.05, **,P < 0.01, ***,P < 0.001 compared with control, Student's_t_ test.
Figure 5
Regulation of AMPA receptor phosphorylation by serotonin (100 μM) in vitro. The amounts of phospho-Ser-831–GluR1 (Left) and phospho-Ser-845–GluR1 (Right) in extracts of slices from WT (black bars) and DARPP-32 KO (white bars) mice were quantified by densitometry. Data represents means ± SE for three to ten experiments. *, P < 0.05, **, P < 0.01, ***, P < 0.001 compared with control; #, P < 0.05, ##, P < 0.01 compared with serotonin in WT, Student's t test.
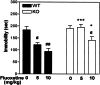
Figure 6
Involvement of DARPP-32 in fluoxetine-mediated immobility in the tail-suspension test for antidepressant efficacy. WT and DARPP-32 KO mice were injected with fluoxetine (5 or 10 mg/kg) 30 min before the trial. The trial was conducted for a period of 5 min, during which the duration of immobility was recorded. Data represents means ± SE for six to ten mice per experiment. #, P < 0.05, ##, P < 0.01 compared with saline; *,P < 0.05, ***,P < 0.001 compared with fluoxetine in DARPP-32 WT mice, two-way ANOVA followed by Duncan's test.
Similar articles
- Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A.
Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. Nishi A, et al. J Neurochem. 2002 May;81(4):832-41. doi: 10.1046/j.1471-4159.2002.00876.x. J Neurochem. 2002. PMID: 12065642 - DARPP-32 mediates serotonergic neurotransmission in the forebrain.
Svenningsson P, Tzavara ET, Liu F, Fienberg AA, Nomikos GG, Greengard P. Svenningsson P, et al. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):3188-93. doi: 10.1073/pnas.052712699. Proc Natl Acad Sci U S A. 2002. PMID: 11880652 Free PMC article. - Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, by casein kinase I in vitro and in vivo.
Desdouits F, Cohen D, Nairn AC, Greengard P, Girault JA. Desdouits F, et al. J Biol Chem. 1995 Apr 14;270(15):8772-8. doi: 10.1074/jbc.270.15.8772. J Biol Chem. 1995. PMID: 7721783 - The role of DARPP-32 in the actions of drugs of abuse.
Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. Nairn AC, et al. Neuropharmacology. 2004;47 Suppl 1:14-23. doi: 10.1016/j.neuropharm.2004.05.010. Neuropharmacology. 2004. PMID: 15464122 Review. - The phosphorylation state of DARPP-32, a third messenger for dopamine, is regulated by in vivo pharmacological treatments.
Di Luca M, Cimino M, Cattabeni F. Di Luca M, et al. Neurochem Int. 1992 Mar;20 Suppl:165S-170S. doi: 10.1016/0197-0186(92)90232-g. Neurochem Int. 1992. PMID: 1365418 Review.
Cited by
- Direct retino-raphe projection alters serotonergic tone and affective behavior.
Ren C, Luan L, Wui-Man Lau B, Huang X, Yang J, Zhou Y, Wu X, Gao J, Pickard GE, So KF, Pu M. Ren C, et al. Neuropsychopharmacology. 2013 Jun;38(7):1163-75. doi: 10.1038/npp.2013.35. Epub 2013 Jan 31. Neuropsychopharmacology. 2013. PMID: 23370156 Free PMC article. - Ca2+/calmodulin-dependent protein kinase II inhibitors disrupt AKAP79-dependent PKC signaling to GluA1 AMPA receptors.
Brooks IM, Tavalin SJ. Brooks IM, et al. J Biol Chem. 2011 Feb 25;286(8):6697-706. doi: 10.1074/jbc.M110.183558. Epub 2010 Dec 14. J Biol Chem. 2011. PMID: 21156788 Free PMC article. - Genetic, pharmacological and lesion analyses reveal a selective role for corticohippocampal GLUN2B in a novel repeated swim stress paradigm.
Kiselycznyk C, Svenningsson P, Delpire E, Holmes A. Kiselycznyk C, et al. Neuroscience. 2011 Oct 13;193:259-68. doi: 10.1016/j.neuroscience.2011.06.015. Epub 2011 Jun 23. Neuroscience. 2011. PMID: 21704131 Free PMC article. - Energizing effects of bupropion on effortful behaviors in mice under positive and negative test conditions: modulation of DARPP-32 phosphorylation patterns.
Carratalá-Ros C, Olivares-García R, Martínez-Verdú A, Arias-Sandoval E, Salamone JD, Correa M. Carratalá-Ros C, et al. Psychopharmacology (Berl). 2021 Dec;238(12):3357-3373. doi: 10.1007/s00213-021-05950-4. Epub 2021 Sep 9. Psychopharmacology (Berl). 2021. PMID: 34498115 Free PMC article. - The Glutamatergic System in Treatment-Resistant Depression and Comparative Effectiveness of Ketamine and Esketamine: Role of Inflammation?
Halaris A, Cook J. Halaris A, et al. Adv Exp Med Biol. 2023;1411:487-512. doi: 10.1007/978-981-19-7376-5_21. Adv Exp Med Biol. 2023. PMID: 36949323
References
- Duman R S, Heninger G R, Nestler E J. Arch Gen Psychiatry. 1997;54:597–606. - PubMed
- Manji H K, Drevets W C, Charney D S. Nat Med. 2001;7:541–547. - PubMed
- Horowski R, Sastre-Y-Hernandez M. Curr Ther Res. 1985;38:23–29.
- Fleischhacker W W, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, Wilf R, Gerlach W, Jaklitsch H, Sastre-Y-Hernandez M, et al. Neuropsychobiology. 1992;26:59–64. - PubMed
- Walaas S I, Aswad D W, Greengard P. Nature (London) 1983;301:69–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases