Ligand binding to somatostatin receptors induces receptor-specific oligomer formation in live cells - PubMed (original) (raw)
Ligand binding to somatostatin receptors induces receptor-specific oligomer formation in live cells
Ramesh C Patel et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Heptahelical receptors (HHRs) are generally thought to function as monomeric entities. Several HHRs such as somatostatin receptors (SSTRs), however, form homo- and heterooligomers when activated by ligand binding. By using dual fluorescent ligands simultaneously applied to live cells monotransfected with SSTR5 (R5) or SSTR1 (R1), or cotransfected with R5 and R1, we have analyzed the ligand receptor stoichiometry and aggregation states for the three receptor systems by fluorescence resonance energy transfer and fluorescence correlation spectroscopy. Both homo- and heterooligomeric receptors are occupied by two ligand molecules. We find that monomeric, homooligomeric, and heterooligomeric receptor species occur in the same cell cotransfected with two SSTRs, and that oligomerization of SSTRs is regulated by ligand binding by a selective process that is restricted to some (R5) but not other (R1) SSTR subtypes. We propose that induction by ligand of different oligomeric states of SSTRs represents a unique mechanism for generating signaling specificity not only within the SSTR family but more generally in the HHR family.
Figures
Figure 1
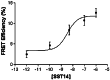
pbFRET analysis of R5/R1 receptors. Dose-dependent increase in effective FRET efficiency induced by treatment with SST-14 of CHO-K1 cells coexpressing HA-R5 and wild-type R1. CHO-K1 cells were treated with increasing concentrations of SST-14 for 30 min at 37°C and analyzed for pbFRET by using FITC-labeled mouse monoclonal anti-HA Abs and rabbit polyclonal R1 Ab directed against the receptor N-terminal segment followed by reaction with rhodamine-conjugated secondary Ab. Thirty to forty cells were analyzed for each experimental condition.
Figure 2
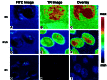
FRET analysis of dual-fluorescent SST ligands interacting with R5, R5/R1, and R1 in live cells. The FITC fluorescence emission (Left), TR fluorescence emission (Center), and GP image (Right) are shown for CHO-K1 cells individually transfected with R5 (Top), coexpressing R5/R1 (Middle), or R1 (Bottom). The GP image was calculated on a pixel by pixel basis from the FITC and TR images as described (18). The R5 and R5/R1 cells were measured by using single-photon excitation, and the R1 cells were measured with two-photon excitation. [Bar = 10 μm (A_–_C) and 25 μm (D_–_I).]
Figure 3
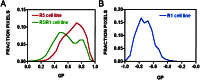
Calculated FRET efficiencies for R5, R5/R1, and R1 cell lines. Normalized histograms of GP values occurring in the cell portion of the GP images in Fig. 2_C_, F, and_I_ are plotted. The cellular portion of the image was determined by visual assessment. The GP value is a measure of fractional intensity. A GP value of −1, 0, and 1 corresponds to signal entirely in the FITC channel, equally split between the two channels, or entirely in the TR channel, respectively. (A) Histogram of the R5 (red line)- and R5/R1 (green line)-transfected cell lines. Only pixels above a certain threshold intensity in the TR channel were included in the histogram to investigate the cell portion of the GP images. (B) Histogram of GP values for R1-transfected cell lines.
Figure 4
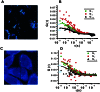
Theoretical auto- and crosscorrelation FCS curves for monomeric and dimeric receptor species derived by using a Monte Carlo in a Grid simulation adapted to FCS measurements (using the SIMFCS program written by E.G.). The two-channel autocorrelation curves shown in green and red are coincident because the number of particles simulated was equally divided between the two species. (A) For the monomer case, the ratio of G12 (0) (crosscorrelation curve shown in black) to G1 (0) should theoretically be zero but shows a finite minimum value because of the crosstalk between the two channels. (B) The curves for the dimeric case reveal the maximum possible ratio of G12 (0) to G1 (0). These two scenarios set the minimum and maximum ratios for the set of experimental conditions simulated.
Figure 5
Experimentally derived auto- and crosscorrelation curves from live R1- and R5/R1-expressing CHO-K1 cells using dual-color two-photon FCS. (A and C) The spots sampled are marked by a pink cross. (B and_D_) Autocorrelation curves are shown in red and green, and crosscorrelation is shown in black. The R5/R1-expressing cells have a greater crosscorrelation relative to the simulated boundaries (represented by the horizontal blue lines on the y axis) than the R1-expressing cells, indicating a higher level of dimer/oligomer formation.
Similar articles
- Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers.
Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. Rocheville M, et al. J Biol Chem. 2000 Mar 17;275(11):7862-9. doi: 10.1074/jbc.275.11.7862. J Biol Chem. 2000. PMID: 10713101 - Somatostatin and its receptor family.
Patel YC. Patel YC. Front Neuroendocrinol. 1999 Jul;20(3):157-98. doi: 10.1006/frne.1999.0183. Front Neuroendocrinol. 1999. PMID: 10433861 Review. - Characterization of cloned somatostatin receptors SSTR4 and SSTR5.
Raynor K, O'Carroll AM, Kong H, Yasuda K, Mahan LC, Bell GI, Reisine T. Raynor K, et al. Mol Pharmacol. 1993 Aug;44(2):385-92. Mol Pharmacol. 1993. PMID: 8102785 - A single amino acid substitution in somatostatin receptor subtype 5 increases affinity for somatostatin-14.
Ozenberger BA, Hadcock JR. Ozenberger BA, et al. Mol Pharmacol. 1995 Jan;47(1):82-7. Mol Pharmacol. 1995. PMID: 7838136 - Pre-clinical and clinical experiences with novel somatostatin ligands: advantages, disadvantages and new prospects.
Hofland LJ, van der Hoek J, Feelders R, van der Lely AJ, de Herder W, Lamberts SW. Hofland LJ, et al. J Endocrinol Invest. 2005;28(11 Suppl International):36-42. J Endocrinol Invest. 2005. PMID: 16625843 Review.
Cited by
- Pharmacological characterisation of native somatostatin receptors in AtT-20 mouse tumour corticotrophs.
Cervia D, Nunn C, Fehlmann D, Langenegger D, Schuepbach E, Hoyer D. Cervia D, et al. Br J Pharmacol. 2003 May;139(1):109-21. doi: 10.1038/sj.bjp.0705235. Br J Pharmacol. 2003. PMID: 12746229 Free PMC article. - Fluorescent approaches for understanding interactions of ligands with G protein coupled receptors.
Sridharan R, Zuber J, Connelly SM, Mathew E, Dumont ME. Sridharan R, et al. Biochim Biophys Acta. 2014 Jan;1838(1 Pt A):15-33. doi: 10.1016/j.bbamem.2013.09.005. Epub 2013 Sep 18. Biochim Biophys Acta. 2014. PMID: 24055822 Free PMC article. Review. - Dynamic imaging of the sodium phosphate cotransporters.
Blaine J, Lanzano L, Giral H, Caldas Y, Levi M, Gratton E, Moldovan R, Lei T. Blaine J, et al. Adv Chronic Kidney Dis. 2011 Mar;18(2):145-50. doi: 10.1053/j.ackd.2011.02.003. Adv Chronic Kidney Dis. 2011. PMID: 21406299 Free PMC article. - Precision engineering of targeted nanocarriers.
Deci MB, Liu M, Dinh QT, Nguyen J. Deci MB, et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018 Sep;10(5):e1511. doi: 10.1002/wnan.1511. Epub 2018 Feb 13. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018. PMID: 29436157 Free PMC article. Review. - Intramembrane receptor-receptor interactions: a novel principle in molecular medicine.
Fuxe K, Canals M, Torvinen M, Marcellino D, Terasmaa A, Genedani S, Leo G, Guidolin D, Diaz-Cabiale Z, Rivera A, Lundstrom L, Langel U, Narvaez J, Tanganelli S, Lluis C, Ferré S, Woods A, Franco R, Agnati LF. Fuxe K, et al. J Neural Transm (Vienna). 2007 Jan;114(1):49-75. doi: 10.1007/s00702-006-0589-0. Epub 2006 Oct 27. J Neural Transm (Vienna). 2007. PMID: 17066251 Review.
References
- Milligan G. J Cell Sci. 2001;114:1265–1271. - PubMed
- Salahpour A, Angers S, Bouvier M. Trends Endocrinol Metab. 2000;11:163–168. - PubMed
- Marshall F H, Jones K A, Kaupmann K, Bettler B. Trends Pharmacol Sci. 1999;20:396–399. - PubMed
- Herrada G, Dulac C. Cell. 1997;90:763–773. - PubMed
- Matsunami H, Buck L B. Cell. 1997;90:775–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources