Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics - PubMed (original) (raw)
Review
Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics
Anton Eberharter et al. EMBO Rep. 2002 Mar.
Abstract
The organization of eukaryotic chromatin has a major impact on all nuclear processes involving DNA substrates. Gene expression is affected by the positioning of individual nucleosomes relative to regulatory sequence elements, by the folding of the nucleosomal fiber into higher-order structures and by the compartmentalization of functional domains within the nucleus. Because site-specific acetylation of nucleosomal histones influences all three aspects of chromatin organization, it is central to the switch between permissive and repressive chromatin structure. The targeting of enzymes that modulate the histone acetylation status of chromatin, in synergy with the effects mediated by other chromatin remodeling factors, is central to gene regulation.
Figures
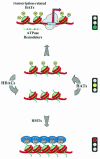
Fig. 1. The histone acetylation switch. Targeted HAT and HDAC activities negotiate the acetylation status of chromatin. Acetylation establishes a structure that permits ATP-dependent chromatin remodeling factors to open promoters. Deacetylation, frequently followed by histone methylation, may form a solid base for highly repressive structures, such as heterochromatin. Acetylated histone tails are shown as yellow circles. Methylations are indicated as gray rectangles. HAT, histone acetyltransferase; HDAC, histone deacetylase; HMT, histone methyltransferase; HP1, heterochromatin protein 1.
Anton Eberharter & Peter B. Becker
Similar articles
- Linking histone acetylation to transcriptional regulation.
Mizzen CA, Allis CD. Mizzen CA, et al. Cell Mol Life Sci. 1998 Jan;54(1):6-20. doi: 10.1007/s000180050121. Cell Mol Life Sci. 1998. PMID: 9487383 Free PMC article. Review. - Role of histone acetylation in the assembly and modulation of chromatin structures.
Annunziato AT, Hansen JC. Annunziato AT, et al. Gene Expr. 2000;9(1-2):37-61. doi: 10.3727/000000001783992687. Gene Expr. 2000. PMID: 11097424 Free PMC article. Review. - Nuclear matrix, dynamic histone acetylation and transcriptionally active chromatin.
Davie JR. Davie JR. Mol Biol Rep. 1997 Aug;24(3):197-207. doi: 10.1023/a:1006811817247. Mol Biol Rep. 1997. PMID: 9291093 Review. - Histone acetylation and chromatin remodeling.
Gregory PD, Wagner K, Hörz W. Gregory PD, et al. Exp Cell Res. 2001 May 1;265(2):195-202. doi: 10.1006/excr.2001.5187. Exp Cell Res. 2001. PMID: 11302684 Review. - Remodeling chromatin structures for transcription: what happens to the histones?
Steger DJ, Workman JL. Steger DJ, et al. Bioessays. 1996 Nov;18(11):875-84. doi: 10.1002/bies.950181106. Bioessays. 1996. PMID: 8939065 Review.
Cited by
- Transcriptional co-activators: emerging roles in signaling pathways and potential therapeutic targets for diseases.
Talukdar PD, Chatterji U. Talukdar PD, et al. Signal Transduct Target Ther. 2023 Nov 13;8(1):427. doi: 10.1038/s41392-023-01651-w. Signal Transduct Target Ther. 2023. PMID: 37953273 Free PMC article. Review. - What Are the Potential Roles of Nuclear Perlecan and Other Heparan Sulphate Proteoglycans in the Normal and Malignant Phenotype.
Hayes AJ, Melrose J. Hayes AJ, et al. Int J Mol Sci. 2021 Apr 23;22(9):4415. doi: 10.3390/ijms22094415. Int J Mol Sci. 2021. PMID: 33922532 Free PMC article. Review. - Aging: All roads lead to mitochondria.
Son JM, Lee C. Son JM, et al. Semin Cell Dev Biol. 2021 Aug;116:160-168. doi: 10.1016/j.semcdb.2021.02.006. Epub 2021 Mar 23. Semin Cell Dev Biol. 2021. PMID: 33741252 Free PMC article. - Methylation-dependent activation of CDX1 through NF-κB: a link from inflammation to intestinal metaplasia in the human stomach.
Rau TT, Rogler A, Frischauf M, Jung A, Konturek PC, Dimmler A, Faller G, Sehnert B, El-Rifai W, Hartmann A, Voll RE, Schneider-Stock R. Rau TT, et al. Am J Pathol. 2012 Aug;181(2):487-98. doi: 10.1016/j.ajpath.2012.04.028. Epub 2012 Jun 27. Am J Pathol. 2012. PMID: 22749770 Free PMC article. - Reprogramming of pancreatic exocrine cells towards a beta (β) cell character using Pdx1, Ngn3 and MafA.
Akinci E, Banga A, Greder LV, Dutton JR, Slack JM. Akinci E, et al. Biochem J. 2012 Mar 15;442(3):539-50. doi: 10.1042/BJ20111678. Biochem J. 2012. PMID: 22150363 Free PMC article.
References
- Akhtar A. and Becker, P.B. (2000) Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell, 5, 367–375. - PubMed
- Anderson J.D., Lowary, P.T. and Widom, J. (2001) Effects of histone acetylation on the equilibrium accessibility of nucleosomal DNA target sites. J. Mol. Biol., 307, 977–985. - PubMed
- Becker P.B. and Hörz, W. (2002) ATP-dependent nucleosome remodeling. Annu. Rev. Biochem., in press. - PubMed
- Berger S.L. (2001) An embarrassment of niches: the many covalent modifications of histones in transcriptional regulation. Oncogene, 20, 3007–3013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases