Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice - PubMed (original) (raw)
Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice
Mikio Furuse et al. J Cell Biol. 2002.
Abstract
The tight junction (TJ) and its adhesion molecules, claudins, are responsible for the barrier function of simple epithelia, but TJs have not been thought to play an important role in the barrier function of mammalian stratified epithelia, including the epidermis. Here we generated claudin-1-deficient mice and found that the animals died within 1 d of birth with wrinkled skin. Dehydration assay and transepidermal water loss measurements revealed that in these mice the epidermal barrier was severely affected, although the layered organization of keratinocytes appeared to be normal. These unexpected findings prompted us to reexamine TJs in the epidermis of wild-type mice. Close inspection by immunofluorescence microscopy with an antioccludin monoclonal antibody, a TJ-specific marker, identified continuous TJs in the stratum granulosum, where claudin-1 and -4 were concentrated. The occurrence of TJs was also confirmed by ultrathin section EM. In claudin-1-deficient mice, claudin-1 appeared to have simply been removed from these TJs, leaving occludin-positive (and also claudin-4-positive) TJs. Interestingly, in the wild-type epidermis these occludin-positive TJs efficiently prevented the diffusion of subcutaneously injected tracer (approximately 600 D) toward the skin surface, whereas in the claudin-1-deficient epidermis the tracer appeared to pass through these TJs. These findings provide the first evidence that continuous claudin-based TJs occur in the epidermis and that these TJs are crucial for the barrier function of the mammalian skin.
Figures
Figure 1.
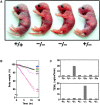
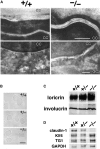
Generation of claudin-1–deficient mice. (A) Restriction maps of the wild-type allele, the targeting vector, and the targeted allele of the mouse claudin-1 gene. The first ATG codon was located in the putative exon 1, which encoded the NH2-terminal portion (amino acids 1–75) of the claudin-1 molecule containing the first transmembrane domain and the first extracellular loop. The targeting vector contained the pgk-neo cassette in its middle portion to delete most of exon 1 in the targeted allele. The position of the probe for Southern blotting is indicated as a bar. E, EcoRI; H, HindIII; S, SacI. (B) Genotype analyses by Southern blotting of HindIII-digested genomic DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice for the mutant claudin-1 gene allele. Southern blotting with the probe indicated in A yielded a 6.8- and 3.9-kb band from the wild-type and targeted allele, respectively. (C) Loss of claudin-1 mRNA in the liver of claudin-1−/− mice examined by RT-PCR. As a control, the hypoxanthine phosphoribosyl transferase gene was equally amplified in all samples. (D) Loss of the claudin-1 protein in the liver of claudin-1−/− mice examined by immunofluorescence microscopy. Frozen sections of the liver were stained with anti–claudin-1 pAb. In the wild-type liver, claudin-1 was concentrated at TJs along bile canaliculi, whereas in the claudin-1−/− liver these signals became undetectable. Bar, 20 μm.
Figure 2.
Impairment of the epidermal barrier in claudin-1–deficient mice. (A) 12-h-old claudin-1+/+, claudin-1+/−, and claudin-1−/− mice. Claudin-1−/− mice were characterized by wrinkled skin and died within 1 d of birth. (B) Dehydration assay. After Cln1 + / − intercross littermates obtained by Caesarian section at embryonic day 18.5 were resuscitated, their weights were monitored hourly without feeding. Genotyping was performed afterwards. Only Cln1 − / − mice showed rapid and steady weight loss. (C) TEWL measurements in two sets of Cln1 + / − intercross newborns. Genotyping identified two Cln1 − / − newborns, and only these mice showed excessive TEWL.
Figure 3.
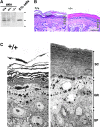
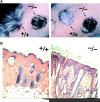
Morphology of the epidermis of claudin-1–deficient mice. (A) Anti–claudin-1 pAb immunoblotting of the whole lysate of the skin obtained from Cln1 + / +, Cln1 + / −, and Cln1 − / − newborn mice. As a control, the cell lysate of L fibroblast transfectants exogenously expressing mouse claudin-1 (C1L cells) was used. Claudin-1 was detected in the Cln1 + / + and Cln1 + / − skin but not in the Cln1 − / − skin. Bars indicate molecular sizes as 31, 21, and 14 kD from the top. (B) Hematoxylin-eosin–stained sectional images of skin obtained from the back of Cln1 + / + and Cln1 − / − newborns. The epidermis of Cln1 − / − newborn mice did not exhibit overt abnormalities in the layered organization of keratinocytes except that stratum corneum appeared to be thicker and compacted tighter than that of the Cln1 + / + epidermis. SC, stratum corneum; GR, stratum granulosum; SP, stratum spinosum; BL, stratum basale. Bar, 20 μm. (C) Ultrathin section electron microscopic images of skin obtained from the back of Cln1 + / + and Cln1 − / − newborns. The appearance of individual keratinocytes was indistinguishable between the Cln1 + / + and Cln1 − / − skin. Bars, 0.5 μm.
Figure 4.
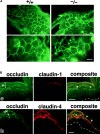
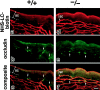
Occludin, claudin-1, and claudin-4 in the wild-type epidermis. (A) Double immunofluorescence microscopy of frozen sections of skin from the back of wild-type mice with antioccludin mAb and anti–claudin-1 pAb. In transverse sections (a and b), occludin was concentrated as dots in the most apical region of the lateral membranes of the granular cells in the second layer (arrowheads). This dot-like concentration of occludin was also found in some but not all of the granular cells in the first (uppermost) and/or third layer. Claudin-1 was distributed diffusely throughout plasma membranes of keratinocytes from stratum basale to granulosum, although the signal from the first layer of stratum granulosum was fairly weak (a). At higher magnification (b), in the lateral membranes of granular cells in the second layer claudin-1 was coconcentrated as dots together with occludin in the most apical regions (arrowheads). In thicker oblique sections (c), claudin-1 was coconcentrated precisely at the occludin-positive lines (arrowhead). SC, stratum corneum. The broken line represents the dermis/ epidermis border. Bars, 10 μm. (B) Double immunofluorescence microscopy of frozen sections of skin from the back of wild-type mice with antioccludin mAb and anti–claudin-4 pAb. Distinct from claudin-1, claudin-4 was distributed mainly in the second/third layers of stratum granulosum (a). Claudin-4 was also concentrated at the occludin-positive lines circumscribing granular cells (arrowhead) (b). SC, stratum corneum. The broken line represents the dermis/epidermis border. Bars, 20 μm.
Figure 6.
Occludin, claudin-1, and claudin-4 in the claudin-1–deficient epidermis. (A) Immunofluorescence microscopy of frozen sections of skin from the back of Cln1 + / + and Cln1 − / − newborn mice with antioccludin mAb. In thick tangential sections of the Cln1 + / + (+/+) and Cln1 − / − (−/−) skin, the occludin signal was detected as continuous lines circumscribing individual granular cells (top). In the Cln1 + / + skin, these honeycomb networks of continuous TJs were occasionally observed in two layers, and this characteristic feature of TJs was not affected by a deficiency of claudin-1 (bottom). Bars, 20 μm. (B) Double immunofluorescence microscopy of frozen sections of skin from the back of Cln1 − / − newborn mice with antioccludin mAb and anti–claudin-1 (a) or anti–claudin-4 pAb (b). As expected, the claudin-1 signal was undetectable, and the distribution of claudin-4 was indistinguishable from that in the Cln1 + / + skin (Fig. 4 B). Occludin-positive TJs (arrowheads). SC, stratum corneum. The broken line represents the dermis/epidermis border. Bars, 20 μm.
Figure 5.
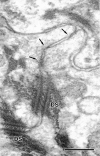
Ultrathin section electron microscopic images of wild-type epidermis. (a and b) Low power electron micrograph (a) of the stratum corneum (SC) and the first through third layers of stratum granulosum (SG1, SG2, and SG3) and a corresponding schematic drawing (b). (c) A boxed area in a and b where occludin was expected to be concentrated was enlarged. Typical TJ (TJ) was detected just above desmosome (DS). (d) Another example of the TJ–desmosome complex observed at the most apical region of the lateral membranes of granular cells in the second layer. Lipid lamellar bodies (arrowheads). Kissing points of TJs (arrows). Bars: (a) 400 nm; (c) 200 nm; (d) 100 nm.
Figure 7.
Ultrathin section electron microscopic image of the claudin-1–deficient epidermis. Similar to Fig. 5, c and d, at the region where occludin was expected to be concentrated TJ-like structures (arrows) were observed frequently just above the desmosome (DS). The structures appeared to be poorly developed compared with those in the wild-type epidermis, although it was technically difficult to quantitatively show this difference by ultrathin section EM. Bar, 200 nm.
Figure 8.
TJ permeability assay of the wild-type and claudin-1–deficient epidermis. An isotonic solution containing freshly made biotinylation reagent into the dermis on the back of Cln1 + / + and Cln1 − / − newborns, and after 30 min incubation the skin was dissected out and frozen. Frozen sections were double stained with antioccludin mAb and streptavidin to label TJs and cross-linked biotin in green and red, respectively. In the Cln1 + / + epidermis (a–c), the biotinylation reagent diffused through the paracellular spaces from stratum basale to the second layer of stratum granulosum, but this diffusion was sharply stopped at occludin-positive TJs (arrows). In the Cln1 − / − epidermis (d–f), the diffusion of injected biotinylation reagent was not prevented at the occludin-positive TJs (arrows). Instead, the biotinylation reagent passed through these TJs to reach the border between stratum granulosum and corneum. SC, stratum corneum. Bar, 10 μm.
Figure 9.
Stratum corneum in claudin-1–deficient mice. (A) Ultrathin section electron microscopic images of ruthenium tetroxide-stained stratum corneum of the skin. Both in the Cln1 + / + and Cln1 − / − epidermis, well-organized lipid lamellae and lamellar bodies were clearly observed between flattened cornified cells (CC; top) and between cornified cells and granular cells (GC; bottom), respectively. Bars, 0.1 μm. (B) Isolated cornified CEs. There was no clear difference in appearance between the Cln1 + / +, Cln1 + / −, and Cln1 − / − skin. Bar, 40 μm. (C) Expression levels of major components of cornified CEs, loricrin and involucrin, detected by immunoblotting. Whole cell lysates of the Cln1 + / +, Cln1 + / −, and Cln1 − / − skin were examined using specific antibodies. (D) Expression levels of Klf4, transglutaminase-1 (TG1), and GAPDH (control) detected by Northern blotting.
Figure 10.
Transplantation of the claudin-1–deficient skin to nude mice. (A) Macroscopic images of the skin grafts on nude mice at 1 mo after transplantation from newborns. The Cln1 + / + grafts bore numerous long hairs, whereas the Cln1 − / − grafts had only a small number of short hairs (left). When hairs were cropped, the Cln1 − / − grafts appeared to be thicker than the Cln1 + / + grafts, and in some places they appeared to be covered with infiltrates (right). (B) Hematoxylin-eosin–stained sectional images of the skin grafts on nude mice at 1 mo after transplantation from newborns. The epidermis of the Cln1 − / − grafts was unusually thick compared with that of the Cln1 + / + grafts, although hair follicles appeared to be developed even in the Cln1 − / − grafts. Bar, 100 μm.
Comment in
- Keratinocyte junctions and the epidermal barrier: how to make a skin-tight dress.
Bazzoni G, Dejana E. Bazzoni G, et al. J Cell Biol. 2002 Mar 18;156(6):947-9. doi: 10.1083/jcb.200202116. Epub 2002 Mar 18. J Cell Biol. 2002. PMID: 11901163 Free PMC article.
Similar articles
- Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice.
Sugawara T, Iwamoto N, Akashi M, Kojima T, Hisatsune J, Sugai M, Furuse M. Sugawara T, et al. J Dermatol Sci. 2013 Apr;70(1):12-8. doi: 10.1016/j.jdermsci.2013.01.002. Epub 2013 Jan 31. J Dermatol Sci. 2013. PMID: 23433550 - Organization and formation of the tight junction system in human epidermis and cultured keratinocytes.
Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I. Brandner JM, et al. Eur J Cell Biol. 2002 May;81(5):253-63. doi: 10.1078/0171-9335-00244. Eur J Cell Biol. 2002. PMID: 12067061 - Tight junction proteins in keratinocytes: localization and contribution to barrier function.
Yuki T, Haratake A, Koishikawa H, Morita K, Miyachi Y, Inoue S. Yuki T, et al. Exp Dermatol. 2007 Apr;16(4):324-30. doi: 10.1111/j.1600-0625.2006.00539.x. Exp Dermatol. 2007. PMID: 17359339 - Tight junctions in epidermis: from barrier to keratinization.
Morita K, Miyachi Y, Furuse M. Morita K, et al. Eur J Dermatol. 2011 Jan-Feb;21(1):12-7. doi: 10.1684/ejd.2010.1192. Eur J Dermatol. 2011. PMID: 21300606 Review. - Tight junctions: is there a role in dermatology?
Kirschner N, Bohner C, Rachow S, Brandner JM. Kirschner N, et al. Arch Dermatol Res. 2010 Sep;302(7):483-93. doi: 10.1007/s00403-010-1058-z. Epub 2010 Jun 19. Arch Dermatol Res. 2010. PMID: 20563589 Review.
Cited by
- Tight Junctions and Cancer: Targeting Claudin-1 and Claudin-4 in Thyroid Pathologies.
Borowczak J, Łaszczych D, Olejnik K, Michalski J, Gutowska A, Kula M, Bator A, Sekielska-Domanowska M, Makarewicz R, Marszałek A, Szylberg Ł, Bodnar M. Borowczak J, et al. Pharmaceuticals (Basel). 2024 Sep 30;17(10):1304. doi: 10.3390/ph17101304. Pharmaceuticals (Basel). 2024. PMID: 39458944 Free PMC article. - Tight junction proteins: from barrier to tumorigenesis.
Runkle EA, Mu D. Runkle EA, et al. Cancer Lett. 2013 Aug 28;337(1):41-8. doi: 10.1016/j.canlet.2013.05.038. Epub 2013 Jun 3. Cancer Lett. 2013. PMID: 23743355 Free PMC article. Review. - Blood-Brain Barrier Protein Claudin-5 Expressed in Paired Xenopus laevis Oocytes Mediates Cell-Cell Interaction.
Brunner N, Stein L, Cornelius V, Knittel R, Fallier-Becker P, Amasheh S. Brunner N, et al. Front Physiol. 2020 Jul 21;11:857. doi: 10.3389/fphys.2020.00857. eCollection 2020. Front Physiol. 2020. PMID: 32848831 Free PMC article. - Prednisolone Targets Claudins in Mouse Brain Blood Vessels.
Markov AG, Bikmurzina AE, Fedorova AA, Vinogradova EP, Kruglova NM, Krivoi II, Amasheh S. Markov AG, et al. Int J Mol Sci. 2023 Dec 24;25(1):276. doi: 10.3390/ijms25010276. Int J Mol Sci. 2023. PMID: 38203447 Free PMC article. - Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection.
Esaki H, Ewald DA, Ungar B, Rozenblit M, Zheng X, Xu H, Estrada YD, Peng X, Mitsui H, Litman T, Suárez-Fariñas M, Krueger JG, Guttman-Yassky E. Esaki H, et al. J Allergy Clin Immunol. 2015 Jan;135(1):153-63. doi: 10.1016/j.jaci.2014.10.037. J Allergy Clin Immunol. 2015. PMID: 25567045 Free PMC article.
References
- Anderson, J.M., and C.M. van Itallie. 1995. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 269:G467–G475. - PubMed
- Balda, M.S., J.A. Whitney, C. Flores, S. González, M. Cereijido, and K. Matter. 1996. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134:1031–1049. - PMC - PubMed
- Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases