The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages - PubMed (original) (raw)
Review
The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages
A K Kiemer et al. Ann Rheum Dis. 2001 Nov.
Abstract
The atrial natriuretic peptide (ANP), a member of the natriuretic peptide family, is a cardiovascular hormone which possesses well defined natriuretic, diuretic, and vasodilating properties. Most of the biological effects of ANP aremediated through its guanylyl cyclase coupled A receptor. Because ANP and its receptors have been shown to be expressed and differentially regulated in the immune system, it has been suggested that ANP has an immunomodulatory potency. Much investigation of the effects of ANP on the activation of macrophages has been carried out. ANP was shown to inhibit the lipopolysaccharide (LPS)-induced expression of inducible nitric oxide synthase (iNOS) in macrophages in an autocrine fashion. ANP in this context was shown to reduce significantly the activation of NF-kappaB and to destabilise iNOS mRNA. ANP, furthermore, can significantly reduce the LPS-induced secretion of tumour necrosis factor alpha (TNFalpha) in macrophages. The relevance of these findings on a regulatory role for ANP on TNFalpha in humans was shown by the fact that ANP significantly reduces the release of TNFalpha in whole human blood. It was furthermore shown to attenuate the release of interleukin 1beta (IL1beta). Interestingly, ANP did not affect the secretion of the anti-inflammatory cytokines IL10 and IL1 receptor antagonist (IL1ra). In summary, ANP was shown to reduce the secretion of inflammatory mediators in macrophages. Therefore, this cardiovascular hormone may possess anti-inflammatory potential.
Figures
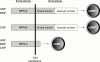
Figure 1
Natriuretic peptide receptors (NPR). The guanylyl cyclases, NPR-A and NPR-B, contain an extracellular ligand binding domain. NPR-A binds ANP and BNP, whereas NPR-B binds CNP. NPR-C has the potency to internalise and clear the natriuretic peptides and exerts other biological effects by inhibiting the production of cAMP.
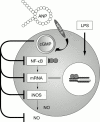
Figure 2
The inhibitory action of ANP on the induction of iNOS. The LPS induction of iNOS is inhibited by ANP through binding to the NPR-A. The inhibitory action involves the destabilisation of iNOS mRNA and inhibition of the activation of NF-κB.
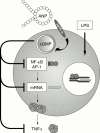
Figure 3
The inhibitory action of ANP on the induction of TNFα. The LPS-induced expression of TNFα is inhibited by ANP through binding to the NPR-A. The inhibitory action involves transcriptional processes with a reduced activation of NF-κB and AP1.
Similar articles
- Autocrine regulation of inducible nitric-oxide synthase in macrophages by atrial natriuretic peptide.
Kiemer AK, Vollmar AM. Kiemer AK, et al. J Biol Chem. 1998 May 29;273(22):13444-51. doi: 10.1074/jbc.273.22.13444. J Biol Chem. 1998. PMID: 9593677 - cGMP-mediated inhibition of TNF-alpha production by the atrial natriuretic peptide in murine macrophages.
Kiemer AK, Hartung T, Vollmar AM. Kiemer AK, et al. J Immunol. 2000 Jul 1;165(1):175-81. doi: 10.4049/jimmunol.165.1.175. J Immunol. 2000. PMID: 10861050 - The atrial natriuretic peptide as a regulator of Kupffer cell functions.
Kiemer AK, Baron A, Gerbes AL, Bilzer M, Vollmar AM. Kiemer AK, et al. Shock. 2002 May;17(5):365-71. doi: 10.1097/00024382-200205000-00004. Shock. 2002. PMID: 12022755 - The role of atrial natriuretic peptide in the immune system.
Vollmar AM. Vollmar AM. Peptides. 2005 Jun;26(6):1086-94. doi: 10.1016/j.peptides.2004.08.034. Epub 2005 Apr 14. Peptides. 2005. PMID: 15911076 Review. - Autocrine and paracrine actions of natriuretic peptides in the heart.
D'Souza SP, Davis M, Baxter GF. D'Souza SP, et al. Pharmacol Ther. 2004 Feb;101(2):113-29. doi: 10.1016/j.pharmthera.2003.11.001. Pharmacol Ther. 2004. PMID: 14761702 Review.
Cited by
- The WNT/Ca2+ pathway promotes atrial natriuretic peptide secretion by activating protein kinase C/transforming growth factor-β activated kinase 1/activating transcription factor 2 signaling in isolated beating rat atria.
Li ZY, Liu Y, Han ZN, Li X, Wang YY, Cui X, Zhang Y. Li ZY, et al. Korean J Physiol Pharmacol. 2022 Nov 1;26(6):469-478. doi: 10.4196/kjpp.2022.26.6.469. Korean J Physiol Pharmacol. 2022. PMID: 36302622 Free PMC article. - The Effects of PPAR Stimulation on Cardiac Metabolic Pathways in Barth Syndrome Mice.
Schafer C, Moore V, Dasgupta N, Javadov S, James JF, Glukhov AI, Strauss AW, Khuchua Z. Schafer C, et al. Front Pharmacol. 2018 Apr 11;9:318. doi: 10.3389/fphar.2018.00318. eCollection 2018. Front Pharmacol. 2018. PMID: 29695963 Free PMC article. - Natriuretic-like Peptide Lebetin 2 Mediates M2 Macrophage Polarization in LPS-Activated RAW264.7 Cells in an IL-10-Dependent Manner.
Bouzazi D, Mami W, Mosbah A, Marrakchi N, Ben Ahmed M, Messadi E. Bouzazi D, et al. Toxins (Basel). 2023 Apr 19;15(4):298. doi: 10.3390/toxins15040298. Toxins (Basel). 2023. PMID: 37104236 Free PMC article. - Factors Associated with Nitric Oxide-mediated β2 Integrin Inhibition of Neutrophils.
Bhopale VM, Yang M, Yu K, Thom SR. Bhopale VM, et al. J Biol Chem. 2015 Jul 10;290(28):17474-84. doi: 10.1074/jbc.M115.651620. Epub 2015 Jun 1. J Biol Chem. 2015. PMID: 26032418 Free PMC article. - Inhibition of prolyl hydroxylases alters cell metabolism and reverses pre-existing diastolic dysfunction in mice.
He X, Zeng H, Roman RJ, Chen JX. He X, et al. Int J Cardiol. 2018 Dec 1;272:281-287. doi: 10.1016/j.ijcard.2018.08.065. Epub 2018 Aug 24. Int J Cardiol. 2018. PMID: 30177233 Free PMC article.
References
- Biochem J. 1985 Dec 1;232(2):313-21 - PubMed
- Cell Mol Life Sci. 1999 Jul;55(8-9):1029-35 - PubMed
- Life Sci. 1989;45(14):1293-7 - PubMed
- Med Res Rev. 1990 Jan-Mar;10(1):115-42 - PubMed
- Endocr Rev. 1989 Nov;10(4):519-36 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources