Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis - PubMed (original) (raw)
Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis
C B Shuster et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Cytokinesis in animal cells is accomplished in part by an actomyosin contractile ring. Recent work on amphibian, Drosophila, and Caenorhabditis elegans embryos implicates membrane trafficking and delivery as essential for cytokinesis. However, the relative contributions of contractile ring constriction versus membrane insertion to cytokinesis and the temporal relationship between these processes are largely unexplored. Here we monitor secretion of the extracellular matrix protein, hyalin, as a marker for new plasma membrane addition in dividing sea urchin zygotes. We find that new membrane addition occurs specifically in the cleavage furrow late in telophase independent of contractile ring constriction. The directed equatorial deposition of new furrow membrane requires astral microtubules and release of internal stores of Ca(2+), but not the presence of a central spindle. Further, cells arrested in M phase do not secrete hyalin, suggesting that mitotic exit is required for new membrane addition. These results demonstrate that astral overlap in equilaterally dividing cells not only serves to specify positioning and contraction of the contractile ring, but also to direct the delivery of new membrane to the furrow as a late, independent event during cytokinesis.
Figures
Figure 1
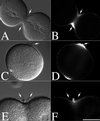
Manipulation of SW composition and cytoskeletal drugs to examine secretion during cell division. L. pictus eggs were fertilized, stripped of their fertilization membranes, and cultured in either normal SW (A) or CaFSW (B) through the first cleavage. Alternatively, cells were cultured in CaFSW until NEB, and then transferred into normal SW (C) or CDSW (D) to inhibit cytokinesis. Additionally, microtubules were disrupted with either 10 μM nocodazole (E) or 50 mM urethane (F). Cells were then fixed and processed for hyalin localization. Arrows denote the accumulation of hyalin at the cleavage plane in D. (Bar = 50 μm.)
Figure 2
Hyalin and membrane accumulation at the cleavage plane of dividing and CD-treated zygotes. L. pictus eggs were fertilized, stripped of their fertilization membranes, and cultured in CaFSW. At NEB, zygotes were transferred to normal SW (A and B) or CDSW (C_–_F). Cells were then fixed and processed for hyalin localization (A_–_D). Alternatively, cells cultured in CaFSW were transferred into CDSW containing 1 μM FM1–43 to directly visualize the plasma membrane (E and F). Arrows denote the position of hyalin. (Bar = 50 μm.)
Figure 3
Positional regulation of secretion by the mitotic apparatus. (A_–_F) Fertilized L. pictus eggs were cultured in CaFSW. Just before NEB, cells were transferred to CDSW. A bent microneedle was gently applied to the surface of the egg, displacing the spindle toward one side of the flattened cell. After anaphase onset (A), the astral microtubules extended toward the cortex (B), and a shallow furrow forms at the midzone between the two aster centers (C). Hyalin appears at the surface during nuclear envelope reformation (D), which accumulates further in the cleavage-arrested cell (E and F) (see Movie 1). Arrows denote the zone of membrane addition. (G_–_I) Just before NEB, cells were cultured on a protamine sulfate-coated coverslip. A bent microneedle was gently applied to the surface of the egg, displacing the spindle toward one side of the flattened cell (G and H). Cells were fixed in place, and after fixation, the needle was removed, and the coverslip was processed for hyalin immunolocalization (I). (Bar = 50 μm.)
Figure 4
Mitotic asters direct both cleavage plane determination and new membrane addition. The first cleavage of L. pictus embryos was suppressed by culturing zygotes in CaFSW containing 50 mM urethane after NEB. Shortly before NEB of the second division, cells were transferred into a chamber containing normal SW, and a glass ball was gently pressed down onto the surface of the egg, displacing the two spindles to opposite sides of the cell. In the absence of cytochalasin (A_–_D), cleavage furrows formed both at the midzone between overlapping asters of the same spindle, as well as between the asters of adjacent spindles. In the presence of 2 μg/ml CD (E_–_H), furrows initiated by overlapping asters over the metaphase plate of each spindle progressed to completion (G). Furrows induced by overlapping asters from different spindles failed to initiate or retracted (G). However, hyalin deposition at these retracted furrows was not affected (H, arrows). Dots indicate the approximate location of the aster centers. The image shown in H represents the region highlighted in G. (Bar = 25 μm.)
Figure 5
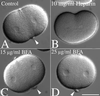
Equatorial hyalin secretion during cell division requires Ca2+-mediated exocytosis but is insensitive to BFA. (A and B) L. pictus zygotes were cultured in CaFSW until NEB, and then transferred into a chamber containing 2 μg/ml CD in normal SW. Cells were then injected with 10 mg/ml heparin, and injected cells were identified by including fluorescein-dextran in the injection buffer (not shown). Cleavage furrows were initiated in both injected and uninjected cells, but the equatorial accumulation of hyalin observed in control cells (A, arrows) was absent in heparin-injected cells (B). (C and D) To determine whether BFA affects hyalin deposition, zygotes were transferred into CaFSW containing 15 or 25 μg/ml BFA (C and D, respectively), and after NEB, transferred into CDSW containing BFA. Arrows denote the equatorial hyalin rings found at the surface of cleavage-arrested cells. (Bar = 50 μm.)
Similar articles
- Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex.
Skop AR, Bergmann D, Mohler WA, White JG. Skop AR, et al. Curr Biol. 2001 May 15;11(10):735-46. doi: 10.1016/s0960-9822(01)00231-7. Curr Biol. 2001. PMID: 11378383 Free PMC article. - Central Spindle Self-Organization and Cytokinesis in Artificially Activated Sea Urchin Eggs.
Henson JH, Buckley MW, Yeterian M, Weeks RM, Simerly CR, Shuster CB. Henson JH, et al. Biol Bull. 2016 Apr;230(2):85-95. doi: 10.1086/BBLv230n2p85. Biol Bull. 2016. PMID: 27132131 - Astral signals spatially bias cortical myosin recruitment to break symmetry and promote cytokinesis.
Werner M, Munro E, Glotzer M. Werner M, et al. Curr Biol. 2007 Aug 7;17(15):1286-97. doi: 10.1016/j.cub.2007.06.070. Curr Biol. 2007. PMID: 17669650 Free PMC article. - How to scaffold the contractile ring for a safe cytokinesis - lessons from Anillin-related proteins.
D'Avino PP. D'Avino PP. J Cell Sci. 2009 Apr 15;122(Pt 8):1071-9. doi: 10.1242/jcs.034785. J Cell Sci. 2009. PMID: 19339546 Review. - Centralspindlin in Rappaport's cleavage signaling.
Mishima M. Mishima M. Semin Cell Dev Biol. 2016 May;53:45-56. doi: 10.1016/j.semcdb.2016.03.006. Epub 2016 Mar 7. Semin Cell Dev Biol. 2016. PMID: 26964770 Review.
Cited by
- A mitotic kinesin-like protein required for normal karyokinesis, myosin localization to the furrow, and cytokinesis in Dictyostelium.
Lakshmikanth GS, Warrick HM, Spudich JA. Lakshmikanth GS, et al. Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16519-24. doi: 10.1073/pnas.0407304101. Epub 2004 Nov 16. Proc Natl Acad Sci U S A. 2004. PMID: 15546981 Free PMC article. - Endosomal recycling controls plasma membrane area during mitosis.
Boucrot E, Kirchhausen T. Boucrot E, et al. Proc Natl Acad Sci U S A. 2007 May 8;104(19):7939-44. doi: 10.1073/pnas.0702511104. Epub 2007 May 1. Proc Natl Acad Sci U S A. 2007. PMID: 17483462 Free PMC article. - The plasma membrane flattens out to fuel cell-surface growth during Drosophila cellularization.
Figard L, Xu H, Garcia HG, Golding I, Sokac AM. Figard L, et al. Dev Cell. 2013 Dec 23;27(6):648-55. doi: 10.1016/j.devcel.2013.11.006. Epub 2013 Dec 5. Dev Cell. 2013. PMID: 24316147 Free PMC article. - The large GTPase dynamin associates with the spindle midzone and is required for cytokinesis.
Thompson HM, Skop AR, Euteneuer U, Meyer BJ, McNiven MA. Thompson HM, et al. Curr Biol. 2002 Dec 23;12(24):2111-7. doi: 10.1016/s0960-9822(02)01390-8. Curr Biol. 2002. PMID: 12498685 Free PMC article. - Breaking up is hard to do - membrane traffic in cytokinesis.
Prekeris R, Gould GW. Prekeris R, et al. J Cell Sci. 2008 May 15;121(Pt 10):1569-76. doi: 10.1242/jcs.018770. J Cell Sci. 2008. PMID: 18469013 Free PMC article.
References
- Wittmann T, Hyman A, Desai A. Nat Cell Biol. 2001;3:E28–E34. - PubMed
- Field C, Li R, Oegema K. Curr Opin Cell Biol. 1999;11:68–80. - PubMed
- Glotzer M. Annu Rev Cell Dev Biol. 2001;17:351–386. - PubMed
- O'Halloran T J. Traffic. 2000;1:921–926. - PubMed
- Straight A F, Field C M. Curr Biol. 2000;10:R760–R770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous