Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties - PubMed (original) (raw)
Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties
Rainer Noiges et al. J Neurosci. 2002.
Abstract
The microtubule-associated proteins 1A (MAP1A) and 1B (MAP1B) are distantly related protein complexes consisting of heavy and light chains and are thought to play a role in regulating the neuronal cytoskeleton, MAP1B during neuritogenesis and MAP1A in mature neurons. To elucidate functional differences between MAP1B and MAP1A and to determine the role of the light chain in the MAP1A protein complex, we chose to investigate the functional properties of the light chain of MAP1A (LC2) and compare them with the light chain of MAP1B (LC1). We found that LC2 binds to microtubules in vivo and in vitro and induces rapid polymerization of tubulin. A microtubule-binding domain in its NH(2) terminus was found to be necessary and sufficient for these activities. The analysis of LC1 revealed that it too bound to microtubules and induced tubulin polymerization via a crucial but structurally unrelated NH(2)-terminal domain. The two light chains differed, however, in their effects on microtubule bundling and stability in vivo. Furthermore, we identified actin filament binding domains located at the COOH terminus of LC2 and LC1 and obtained evidence that binding to actin filaments is attributable to direct interaction with actin. Our findings establish LC2 as a crucial determinant of MAP1A function, reveal LC2 as a potential linker of neuronal microtubules and microfilaments, and suggest that the postnatal substitution of MAP1B by MAP1A leads to expression of a protein with an overlapping but distinct set of functions.
Figures
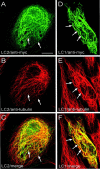
Fig. 1.
The LC2 protein binds to microtubules in vivo. Shown are confocal images of PtK2 cells expressing myc-tagged LC2 (A–C) or myc-tagged LC1 (D–F), analyzed by double immunofluorescence microscopy using antibodies against the myc-tag (A, D) and tubulin (B, E). LC2 and LC1 colocalized with microtubules (arrows), but LC2 did not induce formation of wavy microtubule bundles like LC1 (compare B and_E_). C and F represent merged confocal images of A + B and_D_ + E, respectively. Scale bar, 10 μm.
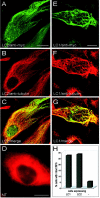
Fig. 2.
Effects of LC2 and LC1 on microtubule stability in cells treated with colchicine. Nontransfected PtK2 cells (NT, D) or PtK2 cells transfected with myc-tagged LC2 (A–C) or myc-tagged LC1 (E–G) were treated with colchicine (1–2 hr, 10 μ
m
) and then analyzed by double immunofluorescence microscopy using antibodies against tubulin (B, D, F) and the myc-tag (A, E). Microtubules were depolymerized in nontransfected cells (D), whereas intact microtubules were found in cells expressing LC2 (B) and LC1 (F).C and G represent merged confocal images of A + B and E +F, respectively. Scale bars, 20 μm. H, One hundred randomly chosen transfected cells were assessed for the presence of intact microtubules (MTs). Quantitative analysis revealed that LC1 and LC2 were equally efficient in protecting microtubules against depolymerization (−, untransfected cells).
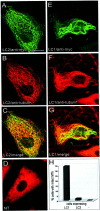
Fig. 3.
Effects of LC2 and LC1 on microtubule stability in cells treated with nocodazole. Nontransfected PtK2 cells (NT, D) or PtK2 cells transfected with myc-tagged LC2 (A–C) or myc-tagged LC1 (E–G) were treated with nocodazole (30–45 min, 10 μg/ml) and then analyzed by double immunofluorescence microscopy using antibodies against tubulin (B, D, F) and the myc-tag (A, E). Microtubules were depolymerized in untransfected cells (D), whereas intact microtubules were found in cells expressing LC2 (B) and LC1 (F).C and G represent merged confocal images of A + B and E +F, respectively. Scale bar, 20 μm. H, One hundred randomly chosen transfected cells were assessed for the presence of intact microtubules (MTs). Quantitative analysis revealed that LC1 was considerably more efficient than LC2 in protecting microtubules against depolymerization (−, untransfected cells).
Fig. 4.
A, Schematic of MAP1A and MAP1B heavy and light chains (HC and LC, respectively) and cDNA constructs used in this study. The scale at the_top_ displays amino acid (aa) residue positions. Domains of sequence homology between MAP1A and MAP1B (hatched boxes) and the microtubule-binding domains (MT) in the heavy and light chains of MAP1B are indicated. The cDNA constructs used are depicted as filled boxes. LC1, Full-length MAP1B light chain (amino acids 2210–2459); N-term LC1, amino acids 2210–2336;C-term LC1, amino acids 2335–2459; LC2, full-length MAP1A light chain (amino acids 2554–2774); N-term LC2, amino acids 2554–2659; C-term LC2, amino acids 2650–2774. For transfection studies, proteins were tagged with an NH2- or COOH-terminal myc peptide. Proteins used for biochemical analysis were tagged with an NH2- or COOH-terminal 6xHis tag. B, LC1 and LC2 interact with microtubules in vitro. LC1 and LC2 proteins (arrows) were sedimented in the presence (+) or absence (−) of polymerized taxol-stabilized microtubules. Equal amounts of supernatant (S) and pellet (P) fractions were analyzed by SDS-PAGE and Coomassie blue staining. Proteins containing the NH2-terminal microtubule-binding domain of LC1 (N-term LC1 and LC1) and LC2 (N-term LC2 and LC2) were found to cosediment with tubulin (T), whereas only trace amounts of the COOH-terminal domains of LC1 (C-term LC1) and LC2 (C-term LC2) were found in the pellet fraction.
Fig. 5.
LC1 and LC2 proteins promote the polymerization of tubulin. LC1 and LC2 proteins were mixed with 1.5 mg/ml tubulin at molar ratios ranging from 1:1.5 to 1:40 as indicated. Polymerization of tubulin was started by placing the mixtures into prewarmed cuvettes and monitored by the change in absorbance at 350 nm. Rapid polymerization of tubulin was observed using the full-length LC1 and LC2 proteins. Microtubule formation in the presence of NH2-terminal domains of LC1 (N-term LC1) and LC2 (N-term LC2) appeared to proceed with a delay; COOH-terminal domains of LC1 (C-term LC1) and tubulin alone (tubulin) were used as controls. The dotted line in each panel defines absorbance level zero.Abs, Absorbance.
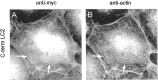
Fig. 6.
The COOH-terminal domain of LC2 interacts with actin stress fibers. PtK2 cells were transfected with the COOH-terminal domain of LC2. Double immunofluorescence microscopy using antibodies against the myc-tag (A) and actin (B) revealed colocalization of the myc-tagged LC2 COOH terminus with actin stress fibers (A and_B_, arrows). Scale bar, 10 μm.
Fig. 7.
The COOH-terminal domains of LC1 and LC2 interact with actin in vitro. Microtiter plates coated with 100 n
m
actin were overlaid with various concentrations of Eu3+-labeled LC1 (A) and LC2 (B) proteins. Full-length LC1 and LC2 as well as their respective COOH-terminal domains (C-term LC1 and_C-term LC2_) showed specific binding to actin. No binding was observed using the NH2-terminal domains of LC1 (N-term LC1) and LC2 (N-term LC2). The amounts of protein bound represent the results of three measurements of at least two independent experiments.
Fig. 8.
LC1 and LC2 cosediment with actin (Ac) via their COOH-terminal domain. LC1 and LC2 proteins (arrows) were sedimented in the presence (+) or absence (−) of polymerized actin. Equal amounts of supernatant (S) and pellet (P) fractions were analyzed by SDS-PAGE and Coomassie blue staining. Full-length LC1 and LC2 were found to cosediment with actin, whereas only trace amounts of the NH2-terminal domains of LC1 (N-term LC1) and LC2 (N-term LC2) were found in the pellet fraction.
Similar articles
- The MAP1 family of microtubule-associated proteins.
Halpain S, Dehmelt L. Halpain S, et al. Genome Biol. 2006;7(6):224. doi: 10.1186/gb-2006-7-6-224. Genome Biol. 2006. PMID: 16938900 Free PMC article. Review. - The functional cooperation of MAP1A heavy chain and light chain 2 in the binding of microtubules.
Chien CL, Lu KS, Lin YS, Hsieh CJ, Hirokawa N. Chien CL, et al. Exp Cell Res. 2005 Aug 15;308(2):446-58. doi: 10.1016/j.yexcr.2005.05.007. Exp Cell Res. 2005. PMID: 15936015 - Heterotypic complex formation between subunits of microtubule-associated proteins 1A and 1B is due to interaction of conserved domains.
Noiges R, Stroissnigg H, Tranciková A, Kalny I, Eichinger R, Propst F. Noiges R, et al. Biochim Biophys Acta. 2006 Oct;1763(10):1011-6. doi: 10.1016/j.bbamcr.2006.08.029. Epub 2006 Aug 25. Biochim Biophys Acta. 2006. PMID: 16996626 - MAP1a associated light chain 3 increases microtubule stability by suppressing microtubule dynamics.
Faller EM, Villeneuve TS, Brown DL. Faller EM, et al. Mol Cell Neurosci. 2009 May;41(1):85-93. doi: 10.1016/j.mcn.2009.02.001. Epub 2009 Feb 20. Mol Cell Neurosci. 2009. PMID: 19233279 - Microtubule-associated protein 1B, a growth-associated and phosphorylated scaffold protein.
Riederer BM. Riederer BM. Brain Res Bull. 2007 Mar 30;71(6):541-58. doi: 10.1016/j.brainresbull.2006.11.012. Epub 2006 Dec 27. Brain Res Bull. 2007. PMID: 17292797 Review.
Cited by
- The MAP1 family of microtubule-associated proteins.
Halpain S, Dehmelt L. Halpain S, et al. Genome Biol. 2006;7(6):224. doi: 10.1186/gb-2006-7-6-224. Genome Biol. 2006. PMID: 16938900 Free PMC article. Review. - Dynamin-like Protein 1 (DNML1) as a Molecular Target for Antibody-Based Immunotherapy to Treat Glaucoma.
Tonner H, Hunn S, Auler N, Schmelter C, Pfeiffer N, Grus FH. Tonner H, et al. Int J Mol Sci. 2022 Nov 7;23(21):13618. doi: 10.3390/ijms232113618. Int J Mol Sci. 2022. PMID: 36362420 Free PMC article. - Epac: effectors and biological functions.
Roscioni SS, Elzinga CR, Schmidt M. Roscioni SS, et al. Naunyn Schmiedebergs Arch Pharmacol. 2008 Jun;377(4-6):345-57. doi: 10.1007/s00210-007-0246-7. Epub 2008 Jan 5. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18176800 Review. - MAP1B Light Chain Modulates Synaptic Transmission via AMPA Receptor Intracellular Trapping.
Palenzuela R, Gutiérrez Y, Draffin JE, Lario A, Benoist M, Esteban JA. Palenzuela R, et al. J Neurosci. 2017 Oct 11;37(41):9945-9963. doi: 10.1523/JNEUROSCI.0505-17.2017. Epub 2017 Sep 13. J Neurosci. 2017. PMID: 28904092 Free PMC article. - Plectin dysfunction in neurons leads to tau accumulation on microtubules affecting neuritogenesis, organelle trafficking, pain sensitivity and memory.
Valencia RG, Mihailovska E, Winter L, Bauer K, Fischer I, Walko G, Jorgacevski J, Potokar M, Zorec R, Wiche G. Valencia RG, et al. Neuropathol Appl Neurobiol. 2021 Feb;47(1):73-95. doi: 10.1111/nan.12635. Epub 2020 Jun 25. Neuropathol Appl Neurobiol. 2021. PMID: 32484610 Free PMC article.
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
- Brugg B, Reddy D, Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligonucleotides inhibits initiation of neurite outgrowth. Neuroscience. 1993;52:489–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases