Listeriolysin O: a genuine cytolysin optimized for an intracellular parasite - PubMed (original) (raw)
Comment
Listeriolysin O: a genuine cytolysin optimized for an intracellular parasite
Shaynoor Dramsi et al. J Cell Biol. 2002.
Abstract
Cholesterol-dependent cytolysins (CDCs)* are produced by a large number of pathogenic gram-positive bacteria. A member of this family, listeriolysin O (LLO), is produced by the intracellular pathogen Listeria monocytogenes. A unique feature of LLO is its low optimal pH activity (approximately 6) which permits escape of the bacterium from the phagosome into the host cell cytosol without damaging the plasma membrane of the infected cell. In a recent study (Glomski et al., 2002, this issue), Portnoy's group has addressed the molecular mechanism underlying the pH sensitivity of LLO. Unexpectedly, a single amino acid substitution in LLO L461T results in a molecule more active at neutral pH and promoting premature permeabilization of the infected cells, leading to attenuated virulence. This finding highlights how subtle changes in proteins can be exploited by bacterial pathogens to establish and maintain the integrity of their specific niches.
Figures
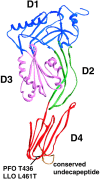
Figure 1.
Structure of perfringolysin with each domain in a different color (in brown the undecapeptide conserved in all CDCs). The position of the amino acid critical for pH sensitivity is highlighted in black (adapted from Rossjohn et al., 1997).
Comment on
- The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells.
Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. Glomski IJ, et al. J Cell Biol. 2002 Mar 18;156(6):1029-38. doi: 10.1083/jcb.200201081. Epub 2002 Mar 18. J Cell Biol. 2002. PMID: 11901168 Free PMC article.
Similar articles
- The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells.
Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. Glomski IJ, et al. J Cell Biol. 2002 Mar 18;156(6):1029-38. doi: 10.1083/jcb.200201081. Epub 2002 Mar 18. J Cell Biol. 2002. PMID: 11901168 Free PMC article. - The chaperone PrsA2 regulates the secretion, stability, and folding of listeriolysin O during Listeria monocytogenes infection.
Agbavor C, Zimnicka A, Kumar A, George JL, Torres M, Prehna G, Alonzo F 3rd, Durrant JD, Freitag NE, Cahoon LA. Agbavor C, et al. mBio. 2024 Jul 17;15(7):e0074324. doi: 10.1128/mbio.00743-24. Epub 2024 May 29. mBio. 2024. PMID: 38809022 Free PMC article. - Irreversible loss of membrane-binding activity of Listeria-derived cytolysins in non-acidic conditions: a distinct difference from allied cytolysins produced by other Gram-positive bacteria.
Nomura T, Kawamura I, Kohda C, Baba H, Ito Y, Kimoto T, Watanabe I, Mitsuyama M. Nomura T, et al. Microbiology (Reading). 2007 Jul;153(Pt 7):2250-2258. doi: 10.1099/mic.0.2007/005843-0. Microbiology (Reading). 2007. PMID: 17600069 - Listeriolysin O: a phagosome-specific lysin.
Schnupf P, Portnoy DA. Schnupf P, et al. Microbes Infect. 2007 Aug;9(10):1176-87. doi: 10.1016/j.micinf.2007.05.005. Epub 2007 May 7. Microbes Infect. 2007. PMID: 17720603 Review. - Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes.
Seveau S. Seveau S. Subcell Biochem. 2014;80:161-95. doi: 10.1007/978-94-017-8881-6_9. Subcell Biochem. 2014. PMID: 24798012 Free PMC article. Review.
Cited by
- Bactofection, Bacterial-Mediated Vaccination, and Cancer Therapy: Current Applications and Future Perspectives.
Renteria-Flores FI, García-Chagollán M, Jave-Suárez LF. Renteria-Flores FI, et al. Vaccines (Basel). 2024 Aug 27;12(9):968. doi: 10.3390/vaccines12090968. Vaccines (Basel). 2024. PMID: 39340000 Free PMC article. Review. - Streptolysin O concentration and activity is central to in vivo phenotype and disease outcome in Group A Streptococcus infection.
Clarke J, Baltazar M, Alsahag M, Panagiotou S, Pouget M, Paxton WA, Pollakis G, Everett D, French N, Kadioglu A. Clarke J, et al. Sci Rep. 2021 Sep 24;11(1):19011. doi: 10.1038/s41598-021-97866-4. Sci Rep. 2021. PMID: 34561464 Free PMC article. - Aptamers: precision tools for diagnosing and treating infectious diseases.
Sujith S, Naresh R, Srivisanth BU, Sajeevan A, Rajaramon S, David H, Solomon AP. Sujith S, et al. Front Cell Infect Microbiol. 2024 Sep 25;14:1402932. doi: 10.3389/fcimb.2024.1402932. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39386170 Free PMC article. Review. - Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view.
Pizarro-Cerdá J, Kühbacher A, Cossart P. Pizarro-Cerdá J, et al. Cold Spring Harb Perspect Med. 2012 Nov 1;2(11):a010009. doi: 10.1101/cshperspect.a010009. Cold Spring Harb Perspect Med. 2012. PMID: 23125201 Free PMC article. Review. - Of inflammasomes and pathogens--sensing of microbes by the inflammasome.
Bauernfeind F, Hornung V. Bauernfeind F, et al. EMBO Mol Med. 2013 Jun;5(6):814-26. doi: 10.1002/emmm.201201771. Epub 2013 May 13. EMBO Mol Med. 2013. PMID: 23666718 Free PMC article. Review.
References
- Bielecki, J., P. Youngman, P. Connelly, and D.A. Portnoy. 1990. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 345:175–176. - PubMed
- Conte, M.P., G. Petrone, C. Longhi, P. Valenti, R. Morelli, F. Superti, and L. Seganti. 1996. The effects of inhibitors of vacuolar acidification on the release of Listeria monocytogenes from phagosomes of Caco-2 cells. J. Med. Microbiol. 44:418–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources