Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population - PubMed (original) (raw)
Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population
Brian P O'Connor et al. J Exp Med. 2002.
Abstract
The contribution that long-lived bone marrow (BM) plasma cells (PCs) provide to enduring humoral immunity has been underscored by a number of recent studies. However, little is known about the immediate precursors that give rise to long-lived PCs in the BM of immune individuals. We have identified subsets of antigen-experienced B cells within the immune BM that are precursors to PCs. These PC precursors arise in the BM 14 days after immunization and persist for greater than 9 months. Phenotypically distinct subsets of PC precursors give rise to short-lived or long-lived PCs. The differentiation of PC precursors to PCs occurs in the absence of antigen and requires cell division. The functional significance of these newly identified PC precursors in the persistence and quality of the humoral immune response is discussed.
Figures
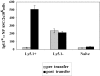
Figure 1.
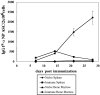
The generation of splenic and BM PCs by Tg αNP-specific B cells. All transfers and immunizations were performed as previously described (16). 3 × 106 Tg cells were transferred to B6-Ly5.2 recipients, with three mice per experimental group. 1 d after transfer, recipient mice were injected intraperitoneally with 100 μg of NP-KLH emulsified in CFA. At 7, 14, 21, and 28 d after immunization, BM and spleen cells were isolated from recipient mice. Splenic and BM αNP-specific IgGa ASC were enumerated by an NP- and allotype-specific ELISPOT assay. ELISPOT assays were performed according to standard Millipore protocol using Millipore HA 96-well plates coated with NP-BSA. Data is representative of four independent experiments.
Figure 2.
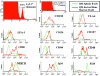
A novel population of Tg B cells in the BM of immune mice that is distinct from naive B cells and PC. Immunization and transfer of Tg B cells to B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM cells were isolated from primary recipients for phenotype analysis. As a comparison to the BM cells, Tg splenocytes from naive mice were also isolated for phenotype analysis. Expression levels for a number of cell surface markers were then determined by three-color flow cytometry as previously described (16). All samples were stained with anti-Ly5.1 and anti-CD138 antibodies. Expression patterns for a number of surface markers on Ly5.1+/CD138+ BM cells or Ly5.1+/CD138low splenocytes were then determined via staining with either anti-VLA4, LFA-1, CD126, CD127, CD80, CD86, CD44, MHCII, B220, or IgM antibodies.
Figure 3.
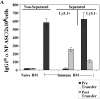
Identification of a Tg PC precursor. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM cells from primary recipients were isolated, depleted of T cells, and then separated by magnetic bead purification for Ly5.1 expression. (A) After separation, 105 Ly5.1+ or Ly5.1− cells were transferred to each secondary recipient B6-Ly5.2 mouse, with three mice per group. 2 wk after transfer, the total of BM cells were isolated from secondary recipients. The levels of αNP IgGa ASC within BM populations were enumerated by αNP- and allotype-specific ELISPOT assays at the time of isolation and postsecondary transfer for separated and unseparated BM populations. ELISPOT assays were performed as described in Fig. 1. Tg B cells express Ly5.1, whereas endogenous host cells express Ly5.2. Data is representative of over 10 independent experiments. (B) After magnetic bead selection, cytospin preparations of the Ly5.1+ cells were stained with Wright-Giemsa. ×1,000.
Figure 3.
Identification of a Tg PC precursor. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM cells from primary recipients were isolated, depleted of T cells, and then separated by magnetic bead purification for Ly5.1 expression. (A) After separation, 105 Ly5.1+ or Ly5.1− cells were transferred to each secondary recipient B6-Ly5.2 mouse, with three mice per group. 2 wk after transfer, the total of BM cells were isolated from secondary recipients. The levels of αNP IgGa ASC within BM populations were enumerated by αNP- and allotype-specific ELISPOT assays at the time of isolation and postsecondary transfer for separated and unseparated BM populations. ELISPOT assays were performed as described in Fig. 1. Tg B cells express Ly5.1, whereas endogenous host cells express Ly5.2. Data is representative of over 10 independent experiments. (B) After magnetic bead selection, cytospin preparations of the Ly5.1+ cells were stained with Wright-Giemsa. ×1,000.
Figure 4.
Functional capacity of BM PCs and precursors early in the immune response. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM cells were isolated from primary recipients and depleted of T cells. (A) Endogenous and Tg PC precursors and PCs within the BM population were then separated by magnetic bead purification for expression of the Ly5.1 (Tg), or (B) Ly5.2 (endogenous) cell surface marker. 105 Ly5.1+ or Ly5.1− cells were transferred to secondary recipients for measurement of Tg-derived ASC, whereas 3 × 106 Ly5.2+ or Ly5.2− cells were transferred to secondary recipients for measurement of endogenous ASC. Three B6-Ly5.2 mice were used per group. Over a 1-mo period after secondary transfer, αNP IgGa ASC levels within the recipient BM population were enumerated by an αNP- and allotype-specific ELISPOT assay. Endogenous αNP IgGb ASC were enumerated at 3 wk after secondary transfer. Data represent the number of ASC found within the donor populations before adoptive transfer as well as posttransfer. ELISPOT assays were performed as in Fig. 1. Data is representative of at least three independent experiments.
Figure 4.
Functional capacity of BM PCs and precursors early in the immune response. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM cells were isolated from primary recipients and depleted of T cells. (A) Endogenous and Tg PC precursors and PCs within the BM population were then separated by magnetic bead purification for expression of the Ly5.1 (Tg), or (B) Ly5.2 (endogenous) cell surface marker. 105 Ly5.1+ or Ly5.1− cells were transferred to secondary recipients for measurement of Tg-derived ASC, whereas 3 × 106 Ly5.2+ or Ly5.2− cells were transferred to secondary recipients for measurement of endogenous ASC. Three B6-Ly5.2 mice were used per group. Over a 1-mo period after secondary transfer, αNP IgGa ASC levels within the recipient BM population were enumerated by an αNP- and allotype-specific ELISPOT assay. Endogenous αNP IgGb ASC were enumerated at 3 wk after secondary transfer. Data represent the number of ASC found within the donor populations before adoptive transfer as well as posttransfer. ELISPOT assays were performed as in Fig. 1. Data is representative of at least three independent experiments.
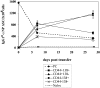
Figure 5.
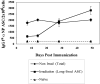
Kinetics of appearance of short-lived and long-lived Tg PCs after immunization. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. Primary recipients were irradiated with 600 rads at 7, 14, 28, or 49 d after transfer of Tg B cells and immunization. Irradiation protocols were performed as stated in Materials and Methods and as previously described (3). 3 wk after irradiation, BM cells were isolated from primary recipients. Tg αNP IgGa ASCs within the BM populations were enumerated by αNP- and allotype-specific ELISPOT assay. ELISPOT assays were performed as described in Fig. 1. Data is representative of two independent experiments.
Figure 6.
Proliferation is required for the differentiation of precursors to PCs early in the immune response. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM was isolated from primary recipients and depleted of T cells. (A) PC precursors and PCs were then separated by magnetic bead purification for Ly5.1 expression and a portion of each fraction was treated with MitoC. MitoC treatment protocol was derived from methods previously described (3). Each population was then transferred separately to B6-Ly5.2 secondary recipients. 105 cells were transferred to each recipient. 2 wk after secondary transfer, BM cells were isolated from secondary recipients. αNP IgGa ASCs within the BM population were then enumerated by αNP- and allotype-specific ELISPOT assay. ELISPOT assays were performed as described in Fig. 1. (B) After Ly5.1 isolation, PC precursors were labeled with CFDAse and transferred (106 per mouse) to B6-Ly5.2 secondary recipients. 3 d after transfer, BM from recipient mice was stained with anti-Ly5.1, and CFDAse expression of the Ly5.1+ cells was determined via FACS® analysis. Data is representative of two independent experiments.
Figure 6.
Proliferation is required for the differentiation of precursors to PCs early in the immune response. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM was isolated from primary recipients and depleted of T cells. (A) PC precursors and PCs were then separated by magnetic bead purification for Ly5.1 expression and a portion of each fraction was treated with MitoC. MitoC treatment protocol was derived from methods previously described (3). Each population was then transferred separately to B6-Ly5.2 secondary recipients. 105 cells were transferred to each recipient. 2 wk after secondary transfer, BM cells were isolated from secondary recipients. αNP IgGa ASCs within the BM population were then enumerated by αNP- and allotype-specific ELISPOT assay. ELISPOT assays were performed as described in Fig. 1. (B) After Ly5.1 isolation, PC precursors were labeled with CFDAse and transferred (106 per mouse) to B6-Ly5.2 secondary recipients. 3 d after transfer, BM from recipient mice was stained with anti-Ly5.1, and CFDAse expression of the Ly5.1+ cells was determined via FACS® analysis. Data is representative of two independent experiments.
Figure 7.
PC precursors are long-lived. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. BM was isolated from primary recipients 9 mo after immunization and depleted of T cells. PC precursors and PCs were then separated by magnetic bead purification for Ly5.1 expression and transferred separately to B6-Ly5.2 secondary recipients. 6 × 104 PC precursors or PCs were transferred to secondary recipients. 2 mo after secondary transfer, BM cells from secondary recipients were isolated. αNP IgGa ASC levels within the BM were measured by an αNP- and allotype-specific ELISPOT assay both before and postsecondary transfer. ELISPOT assays were performed as described in Fig. 1. Data is representative of two independent experiments.
Figure 8.
Phenotypic and functional diversity of PC precursor subsets. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. PCs and PC precursors were isolated from primary recipient BM 2 wk after immunization via Ly5.1 magnetic bead selection. The precursor fraction was then further separated via FACS® sorting for expression of CD44 and CD138 by fluorescent antibody staining. Each population of sorted precursor cells and PCs were transferred separately to B6-Ly5.2 secondary recipients. 4 × 104 cells were transferred to each recipient. BM cells were isolated from secondary recipients over a 1-mo period after transfer. αNP IgGa ASC were enumerated by an αNP-, allotype-specific ELISPOT assay. ELISPOT assays were performed as described in Fig. 1. Data is representative of four independent experiments.
Similar articles
- Lasting antibody responses are mediated by a combination of newly formed and established bone marrow plasma cells drawn from clonally distinct precursors.
Chernova I, Jones DD, Wilmore JR, Bortnick A, Yucel M, Hershberg U, Allman D. Chernova I, et al. J Immunol. 2014 Nov 15;193(10):4971-9. doi: 10.4049/jimmunol.1401264. Epub 2014 Oct 17. J Immunol. 2014. PMID: 25326027 Free PMC article. - In vitro generation of long-lived human plasma cells.
Cocco M, Stephenson S, Care MA, Newton D, Barnes NA, Davison A, Rawstron A, Westhead DR, Doody GM, Tooze RM. Cocco M, et al. J Immunol. 2012 Dec 15;189(12):5773-85. doi: 10.4049/jimmunol.1103720. Epub 2012 Nov 16. J Immunol. 2012. PMID: 23162129 - Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice.
Lemke A, Kraft M, Roth K, Riedel R, Lammerding D, Hauser AE. Lemke A, et al. Mucosal Immunol. 2016 Jan;9(1):83-97. doi: 10.1038/mi.2015.38. Epub 2015 May 6. Mucosal Immunol. 2016. PMID: 25943272 - The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease.
O'Connor BP, Gleeson MW, Noelle RJ, Erickson LD. O'Connor BP, et al. Immunol Rev. 2003 Aug;194:61-76. doi: 10.1034/j.1600-065x.2003.00055.x. Immunol Rev. 2003. PMID: 12846808 Free PMC article. Review. - Determinations of B cell fate in immunity and autoimmunity.
Noelle RJ, Erickson LD. Noelle RJ, et al. Curr Dir Autoimmun. 2005;8:1-24. doi: 10.1159/000082084. Curr Dir Autoimmun. 2005. PMID: 15564715 Review.
Cited by
- Successive Oral Immunizations Against Piscirickettsia Salmonis and Infectious Salmon Anemia Virus are Required to Maintain a Long-Term Protection in Farmed Salmonids.
Tobar I, Arancibia S, Torres C, Vera V, Soto P, Carrasco C, Alvarado M, Neira E, Arcos S, Tobar JA. Tobar I, et al. Front Immunol. 2015 May 27;6:244. doi: 10.3389/fimmu.2015.00244. eCollection 2015. Front Immunol. 2015. PMID: 26074916 Free PMC article. - New Insights into the Treatment of Glomerular Diseases: When Mechanisms Become Vivid.
Lin DW, Chang CC, Hsu YC, Lin CL. Lin DW, et al. Int J Mol Sci. 2022 Mar 24;23(7):3525. doi: 10.3390/ijms23073525. Int J Mol Sci. 2022. PMID: 35408886 Free PMC article. Review. - Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI.
Wolf AI, Mozdzanowska K, Quinn WJ 3rd, Metzgar M, Williams KL, Caton AJ, Meffre E, Bram RJ, Erickson LD, Allman D, Cancro MP, Erikson J. Wolf AI, et al. J Clin Invest. 2011 Oct;121(10):3954-64. doi: 10.1172/JCI57362. Epub 2011 Sep 1. J Clin Invest. 2011. PMID: 21881204 Free PMC article. - mRNA-Based Vaccine Designing against Epstein-Barr Virus to Induce an Immune Response Using Immunoinformatic and Molecular Modelling Approaches.
Althurwi HN, Alharthy KM, Albaqami FF, Altharawi A, Javed MR, Muhseen ZT, Tahir Ul Qamar M. Althurwi HN, et al. Int J Environ Res Public Health. 2022 Oct 11;19(20):13054. doi: 10.3390/ijerph192013054. Int J Environ Res Public Health. 2022. PMID: 36293632 Free PMC article. - Divide and conquer: the importance of cell division in regulating B-cell responses.
Tangye SG, Hodgkin PD. Tangye SG, et al. Immunology. 2004 Aug;112(4):509-20. doi: 10.1111/j.1365-2567.2004.01950.x. Immunology. 2004. PMID: 15270721 Free PMC article. Review.
References
- Manz, R.A., M. Lohning, G. Cassese, A. Thiel, and A. Radbruch. 1998. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 10:1703–1711. - PubMed
- Maruyama, M., K.P. Lam, and K. Rajewsky. 2000. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 407:636–642. - PubMed
- Slifka, M.K., R. Antia, J.K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity. 8:363–372. - PubMed
- McHeyzer-Williams, M.G., and R. Ahmed. 1999. B cell memory and the long-lived plasma cell. Curr. Opin. Immunol. 11:172–179. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources