Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation - PubMed (original) (raw)
Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation
Benjamin R tenOever et al. J Virol. 2002 Apr.
Erratum in
- J Virol 2002 Jun;76(12):6413
Abstract
The mechanisms of cellular recognition for virus infection remain poorly understood despite the wealth of information regarding the signaling events and transcriptional responses that ensue. Host cells respond to viral infection through the activation of multiple signaling cascades, including the activation of NF-kappaB, c-Jun/ATF-2 (AP-1), and the interferon regulatory factors (IRFs). Although viral products such as double-stranded RNA (dsRNA) and the processes of viral binding and fusion have been implicated in the activation of NF-kappaB and AP-1, the mechanism(s) of IRF-1, IRF-3, and IRF-7 activation has yet to be fully elucidated. Using recombinant measles virus (MeV) constructs, we now demonstrate that phosphorylation-dependent IRF-3 activation represents a novel cellular detection system that recognizes the MeV nucleocapsid structure. At low multiplicities of infection, IRF-3 activation is dependent on viral transcription, since UV cross-linking and a deficient MeV containing a truncated polymerase L gene failed to induce IRF-3 phosphorylation. Expression of the MeV nucleocapsid (N) protein, without the requirement for any additional viral proteins or the generation of dsRNA, was sufficient for IRF-3 activation. In addition, the nucleocapsid protein was found to associate with both IRF-3 and the IRF-3 virus-activated kinase, suggesting that it may aid in the colocalization of the kinase and the substrate. Altogether, this study suggests that IRF-3 recognizes nucleocapsid structures during the course of an MeV infection and triggers the induction of interferon production.
Figures
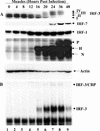
FIG. 1.
Activation of IRFs following MeV infection in A549 cells. (A) Whole-cell extract (100 μg) prepared from A549 cells infected with MeV (MOI = 1.0) for different periods of time was resolved by SDS-7.5% PAGE and transferred to nitrocellulose. IRF-3 (forms I to IV), IRF-7, IRF-1, MeV proteins, and actin were detected by immunoblotting. MeV phosphoprotein, hemagglutinin protein, and nucleocapsid protein are denoted P, H, and N, respectively. (B) Nuclear extracts were collected from duplicate experiments as outlined above and used to analyze IRF-3 binding activity by electrophoretic mobility shift assay using the ISRE of ISG15 as the probe. Arrows indicate complexes of IRF-3 and IRF-3/CBP.
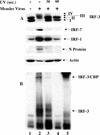
FIG. 2.
IRF-3 activation requires viral transcription. (A) MeV stocks were UV treated for 0, 30, or 60 s and added to target A549 cells at an MOI of 1.0 for 24 h. Whole-cell extract (75 μg) was prepared and run on SDS-7.5% PAGE. Endogenous proteins were detected by immunoblotting with IRF-3, IRF-7, IRF-1, MeV N, and actin antibodies. (B) Nuclear extracts were prepared from duplicate experiments as outlined above and used to analyze IRF-3 DNA binding activity by electrophoretic mobility shift assay using the ISRE of ISG15 as the probe. Arrows indicate complex formation of IRF-3 and IRF-3/CBP as determined by supershift of these complexes (IRF-3 supershift, lane 5; CBP supershift, data not shown).
FIG. 3.
IRF-3 activation requires a functional MeV polymerase. (A) Rescued MeV from wild-type cDNA (MeVwt) or cDNA encoding a truncated polymerase (MeVΔL) was produced in 293-3-46 cells and used to treat target A549 cells as described in the Materials and Methods section. At 72 h.p.i., cells were harvested and run on an SDS-7.5% PAGE gel. Endogenous proteins were detected in whole-cell extract (100 μg) by immunoblotting with IRF-3, IRF-7, IRF-1, MeV, and actin antibodies. (B) Nuclear extracts were collected from duplicate experiments as outlined above and used to analyze IRF-3 DNA binding activity by electrophoretic mobility shift assay using the ISRE of ISG15 as the probe. Arrows indicate complex formation of IRF-3 and IRF-3/CBP as determined by supershift of these complexes (IRF-3 supershift, lane 4; CBP supershift, data not shown).
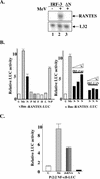
FIG. 4.
RANTES induction by MeV and MeV N protein. (A) HEK293 cells expressing IRF-3 ΔN and IRF-3 wild-type were infected with MeV (MOI = 1.0) for 72 h. Total RNA (5 μg) was isolated and analyzed by RPA for the expression of RANTES and L32. (B) Left panel: HEK293 cells were transfected with the κB-mutated RANTES promoter (κBm-RANTES-LUC) reporter plasmids and MeV protein constructs as indicated. At 24 h posttransfection, cells were harvested, and relative luciferase (LUC) activity was measured as fold activation. Each value represents the mean ± standard error (SE) of triplicate determinations. Right panel: HEK293 cells were cotransfected with increasing concentrations of N cDNA (0.5 to 1.5 μg) in addition to a constant level of either IRF-3 wild-type or IRF-3 ΔN (1.0 μg). Experiment was performed as above. (C) HEK293 cells were transfected with the NF-κB-responsive promoter P2 (2) and either empty vector or N protein. Cells were treated with MeV (MOI = 1.0) or dsRNA (100 μg/ml) and harvested 36 h posttransfection, and relative luciferase activity was measured as fold activation. Each value represents the mean ± SE of quadruplicate experiments. The treatments and constructs used were: untreated (U), MeV (Me), nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin protein (H), large polymerase protein (P), and dsRNA.
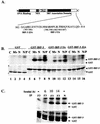
FIG. 5.
Nucleocapsid protein alone induces IRF-3 phosphorylation. (A) HEK293 cells in 10-cm plates were cotransfected with Myc-IRF-3 (5 μg) and increasing amounts of Flag-N (5 to 15 μg; lanes 3 to 5). At 36 h posttransfection or 24 h.p.i. with MeV (MOI = 1.0), whole-cell extract (15 μg) was run on SDS-7.5% PAGE and analyzed by immunoblots using Flag and Myc antibodies. The positions of IRF-3 forms I, II, and III are indicated. (B) HEK293 cells in 10-cm plates were cotransfected with empty vector or Flag-N and Myc-IRF-3. Sendai virus (SeV) infection was performed at 25 HAU/106 cells for 10 h. At 36 h posttransfection, cells were harvested and subjected to two-dimensional electrophoresis. Shifts to the right imply phosphorylation events. (C) Stable cells expressing Flag N or untagged N and P (293-3-46) were transfected with Flag-IRF-3 (5 μg). At 36 h posttransfection, whole-cell extract (15 μg) was run on SDS-7.5% PAGE and analyzed by immunoblot using Flag antibody. Immunoblots for IRF-1 and IRF-7 were determined from whole-cell extract (80 μg) run on SDS-10% PAGE. (D) Nuclear extracts were collected from duplicate experiments as outlined above and used to analyze IRF-3 DNA binding activity by electrophoretic mobility shift assay using the ISRE of ISG15 as the probe. Arrows indicate complex formation of IRF-3 as determined by supershift analysis (IRF-3 supershift, lanes 3 and 6; CBP supershift, not shown).
FIG. 6.
Nucleocapsid protein induces and associates with the virus activated kinase (VAK). (A) Schematic representation of IRF-3. The DNA binding domain, the nuclear export sequence element, the proline-rich region, and the C-terminal IRF association domain are indicated. The region between amino acids 382 and 414 is expanded below the schematic in a depiction of IRF-3 C-terminal phosphorylation sites. The construct with serines 385 and 386 mutated to alanine has been denoted IRF-3 J2A. Residues 396, 398, 402, 404, and 405 mutated to alanine have been denoted IRF-3 5A. (B) Whole-cell extract (2 μg) derived from uninfected (−), MeV-infected at 24 h.p.i. at an MOI of 1.0 (Mv), and the stable Flag-N (N) and 293-3-46 (N/P) cell lines were used in an in vitro kinase assay using GST, GST-IRF-3 wild-type, GST-IRF-3 5A, and GST-IRF-3 J2A as substrates In all cases the IRF-3 moiety of the substrate spanned amino acids 380 to 427. Kinase reactions were run on SDS-12% PAGE, stained with Coomassie, dried, and exposed for 1 h at −70°C on an autoradiographic film. (C) HEK293 cells transfected with Flag-IRF-3 or Flag-N and harvested 36 h posttransfection. Sendai virus (SeV) infections were performed at the time points indicated. Whole-cell extract (1 mg) was incubated with protein G and Flag antibody overnight, and a kinase assay was performed as described for panel B with what was retained following the washes.
FIG. 7.
Characterization of N and IRF-3 interaction. (A) HEK293 cells stably expressing Flag-N in 10-cm plates were transfected with various Myc-IRF-3 constructs encoding the various IRF-3 deletions depicted above in the schematic. Following immunoprecipitation of Flag-N, complexes were run on SDS-12% PAGE and immunoblotted using the anti-Myc antibody. Wild-type IRF-3 (amino acids 1 to 427; lanes 1 and 5), IRF-3 lacking the autoinhibitory domain (amino acids 1 to 394; lanes 2 and 6), IRF-3 lacking the IRF association domain (amino acids 1 to 197; lanes 3 and 7), and IRF-3 lacking the nuclear export sequence and proline-rich region (PRO) (Δ134-197; lanes 4 and 8) are depicted. The 10% input consisted of 50 μg of whole-cell extract (lanes 1 to 4). (B) HEK293 cells were cotransfected with Myc-IRF-3 and various Flag-N deletion constructs depicted in the schematic. Wild-type Flag-N (amino acids 1 to 523; lanes 1 and 5), two C-terminal deletions, Flag-N (amino acids 1 to 375; lanes 2 and 6) and Flag-N (amino acids 1 to 415; lanes 3 and 7), and an N-terminal mutant, Flag-N (amino acids 134 to 523; lanes 4 and 8), are depicted. The 10% input consisted of 50 μg of whole-cell extract (lanes 5 to 8).
FIG. 8.
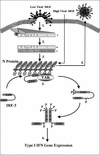
Schematic representation of IRF-3 activation following MeV infection. Following viral binding and fusion, the genome of MeV is released into the cytoplasm in tight association with the nucleocapsid (N) protein. Upon entry, N dissociates from the negative-stranded template as it is transcribed by the packaged polymerase composed of both the large (L) and phospho- (P) proteins of MeV (step 1). Transcription induces a gradient of protein production transcribing the highest amounts of the 3′ N gene and the lowest amount of the 5′-most L gene. As intracellular N protein concentrations rise, N associates with the genome template, causing a switch from transcription to replication by inducing the production of readthrough (+) full-length genome templates (step 2). Positive RNA synthesis serves as template for the production of full-length negative genomes, which, upon synthesis, are tightly bound by the N protein (step 3). Newly synthesized negative-strand genomes, associated with N, bind additional viral proteins in cooperation with numerous cellular proteins, allowing progeny virus to bud from the infected cell (step 4). During the course of infection, IRF-3 physically associates with the N protein of MeV through the interferon association domain (IAD) and its C-terminal negatively charged domain, leading to the C-terminal phosphorylation of IRF-3 by the virus-activated kinase (VAK) (step 5). Phosphorylation of IRF-3 is followed by its release from N, IRF dimerization, nuclear translocation, DNA binding, association with CBP, and transcriptional activation of the IFN-α/β genes (step 6). IAD, IRF association domain; DBD, DNA binding domain; RBD, RNA binding domain; N/P, hydrophobic domain involved in N/P interactions; and [−], negatively charged domain.
Similar articles
- The interaction between the measles virus nucleoprotein and the Interferon Regulator Factor 3 relies on a specific cellular environment.
Colombo M, Bourhis JM, Chamontin C, Soriano C, Villet S, Costanzo S, Couturier M, Belle V, Fournel A, Darbon H, Gerlier D, Longhi S. Colombo M, et al. Virol J. 2009 May 15;6:59. doi: 10.1186/1743-422X-6-59. Virol J. 2009. PMID: 19445677 Free PMC article. - Multiple signaling pathways leading to the activation of interferon regulatory factor 3.
Servant MJ, Grandvaux N, Hiscott J. Servant MJ, et al. Biochem Pharmacol. 2002 Sep;64(5-6):985-92. doi: 10.1016/s0006-2952(02)01165-6. Biochem Pharmacol. 2002. PMID: 12213596 Review. - Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways.
Iwamura T, Yoneyama M, Yamaguchi K, Suhara W, Mori W, Shiota K, Okabe Y, Namiki H, Fujita T. Iwamura T, et al. Genes Cells. 2001 Apr;6(4):375-88. doi: 10.1046/j.1365-2443.2001.00426.x. Genes Cells. 2001. PMID: 11318879 - Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA.
Servant MJ, Grandvaux N, tenOever BR, Duguay D, Lin R, Hiscott J. Servant MJ, et al. J Biol Chem. 2003 Mar 14;278(11):9441-7. doi: 10.1074/jbc.M209851200. Epub 2003 Jan 10. J Biol Chem. 2003. PMID: 12524442 - Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback.
Levy DE, Marié I, Smith E, Prakash A. Levy DE, et al. J Interferon Cytokine Res. 2002 Jan;22(1):87-93. doi: 10.1089/107999002753452692. J Interferon Cytokine Res. 2002. PMID: 11846979 Review.
Cited by
- Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity.
tenOever BR, Sharma S, Zou W, Sun Q, Grandvaux N, Julkunen I, Hemmi H, Yamamoto M, Akira S, Yeh WC, Lin R, Hiscott J. tenOever BR, et al. J Virol. 2004 Oct;78(19):10636-49. doi: 10.1128/JVI.78.19.10636-10649.2004. J Virol. 2004. PMID: 15367631 Free PMC article. - Cell surface delivery of the measles virus nucleoprotein: a viral strategy to induce immunosuppression.
Marie JC, Saltel F, Escola JM, Jurdic P, Wild TF, Horvat B. Marie JC, et al. J Virol. 2004 Nov;78(21):11952-61. doi: 10.1128/JVI.78.21.11952-11961.2004. J Virol. 2004. PMID: 15479835 Free PMC article. - Bunyamwera virus nonstructural protein NSs counteracts interferon regulatory factor 3-mediated induction of early cell death.
Kohl A, Clayton RF, Weber F, Bridgen A, Randall RE, Elliott RM. Kohl A, et al. J Virol. 2003 Jul;77(14):7999-8008. doi: 10.1128/jvi.77.14.7999-8008.2003. J Virol. 2003. PMID: 12829839 Free PMC article. - Downregulation of mitochondrial biogenesis by virus infection triggers antiviral responses by cyclic GMP-AMP synthase.
Sato H, Hoshi M, Ikeda F, Fujiyuki T, Yoneda M, Kai C. Sato H, et al. PLoS Pathog. 2021 Oct 14;17(10):e1009841. doi: 10.1371/journal.ppat.1009841. eCollection 2021 Oct. PLoS Pathog. 2021. PMID: 34648591 Free PMC article. - Incoming RNA virus nucleocapsids containing a 5'-triphosphorylated genome activate RIG-I and antiviral signaling.
Weber M, Gawanbacht A, Habjan M, Rang A, Borner C, Schmidt AM, Veitinger S, Jacob R, Devignot S, Kochs G, García-Sastre A, Weber F. Weber M, et al. Cell Host Microbe. 2013 Mar 13;13(3):336-46. doi: 10.1016/j.chom.2013.01.012. Cell Host Microbe. 2013. PMID: 23498958 Free PMC article.
References
- Au, W.-C., P. A. Moore, W. Lowther, Y.-T. Juang, and P. M. Pitha. 1995. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. USA 92:11657-11661. - PMC - PubMed
- Banerjee, A. K., S. Barik, and B. P. De. 1991. Gene expression of nonsegmented negative strand RNA viruses. Pharmacol. Ther. 51:47-70. - PubMed
- Blumberg, B. M., M. Leppert, and D. Kolakofsky. 1981. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell 23:837-845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous