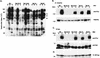The direct recruitment of BLNK to immunoglobulin alpha couples the B-cell antigen receptor to distal signaling pathways - PubMed (original) (raw)
The direct recruitment of BLNK to immunoglobulin alpha couples the B-cell antigen receptor to distal signaling pathways
Shara Kabak et al. Mol Cell Biol. 2002 Apr.
Abstract
Following B-cell antigen receptor (BCR) ligation, the cytoplasmic domains of immunoglobulin alpha (Ig alpha) and Ig beta recruit Syk to initiate signaling cascades. The coupling of Syk to several distal substrates requires linker protein BLNK. However, the mechanism by which BLNK is recruited to the BCR is unknown. Using chimeric receptors with wild-type and mutant Ig alpha cytoplasmic tails we show that the non-immunoreceptor tyrosine-based activation motif (ITAM) tyrosines, Y176 and Y204, are required to activate BLNK-dependent pathways. Subsequent analysis demonstrated that BLNK bound directly to phospho-Y204 and that fusing BLNK to mutated Ig alpha reconstituted downstream signaling events. Moreover, ligation of the endogenous BCR induced Y204 phosphorylation and BLNK recruitment. These data demonstrate that the non-ITAM tyrosines of Ig alpha couple Syk activation to BLNK-dependent pathways.
Figures
FIG. 1.
Expression of the PDGFRβ/Igα chimeric proteins. (A) Chimeric proteins were constructed by fusing the extracellular and transmembrane (TM) domains of PDGFRβ to the cytoplasmic domain of either wild-type or mutated Igα. The mutants contain tyrosine-to-phenylalanine mutations at both ITAM tyrosines (Igα182,193), at both non-ITAM tyrosines (Igα176,204), or at either non-ITAM tyrosine individually (Igα176 and Igα204). (B) B-cell surface expression of BCR (left) and PDGFRβ (right) on transfected cells as measured by flow cytometry. Cells stained with the secondary antibody only were used as a negative control (shaded peaks).
FIG. 2.
Igα176 and Igα204 are required for tyrosine phosphorylation of selected substrates. (A) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated PDGFRβ/Igα chimeras (C) for 2 min. Lysates were analyzed by immunoblotting with antiphosphotyrosine antibodies. Arrows, proteins with decreased tyrosine phosphorylation induced through the mutant chimeras compared to that induced through BCR. (B) Cells were left unstimulated (U) or were stimulated through various PDGFRβ/Igα chimeras (C) for 2 min. Lysates were immunoprecipitated (IP) with anti-PDGFRβ antibodies to precipitate the chimera and immunoblotted with antiphosphotyrosine (anti-PTyr) antibodies (top). The blot was then stripped and reprobed with anti-PDGFRβ antibodies (bottom). (C) Cells were stimulated as for panel B. Lysates were immunoprecipitated with anti-Syk antibodies and immunoblotted with antiphosphotyrosine antibodies (top). The blot was then stripped and reprobed with anti-Syk antibodies (bottom). (D) Cells were stimulated as for panel B. Brij lysates (1%) were immunoprecipitated with anti-Syk antibodies and subjected to a kinase assay using a GST-Igα cytoplasmic tail as an exogenous substrate. After SDS-PAGE, the gel was dried and phosphorylation of GST-Igα was detected by autoradiography.
FIG. 3.
Igα176 and Igα204 are required for the phosphorylation of BLNK and for the activation of distal pathways. (A) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated PDGFRβ/Igα chimeras (C) for 3 min. Lysates were immunoprecipitated (IP) with anti-PLC-γ2 antibodies and immunoblotted with antiphosphotyrosine (anti-PTyr) antibodies (top). The blot was then stripped and reprobed with anti-PLC-γ2 antibodies (bottom). (B) Cells were loaded with Fura red and fluo-3 and stimulated through either the BCR (left) or the indicated chimera (right). The relative mean increase in intracellular calcium is plotted as a function of time. Left arrows, addition of stimulating antibody; right arrows, addition of ionomycin. (C) Cells were stimulated as for panel A. Lysates were immunoprecipitated with anti-BLNK antibodies and immunoblotted with antiphosphotyrosine antibodies (top). The blot was then stripped and reprobed with anti-BLNK antibodies (bottom). (D) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated chimeras (C) for 5 min. Lysates were immunoblotted with anti-phospho-ERK1 and -ERK2 antibodies (top). The blot was then stripped and reprobed with anti-ERK1 and -ERK2 antibodies (bottom). (E) Cells were stimulated as for panel D. Lysates were precipitated with GST-c-Jun and subjected to a kinase assay and SDS-PAGE, followed by detection of phosphorylated c-Jun by autoradiography.
FIG. 4.
BLNK binds directly to phosphorylated Y204 in vivo and in vitro. (A) Eleven-amino-acid peptides spanning Y176 (MPDDYEDENLY) or Y204 (LQGTYQDVGNL) were synthesized with or without a phosphate on the middle tyrosine and covalently coupled to _N_-hydroxy-succinimide-activated Sepharose. Cells were either left unstimulated (U) or were stimulated through the BCR (B) for 2 min. Lysates were precipitated with peptide-coupled beads and immunoblotted with antiphosphotyrosine (anti-PTyr) antibodies (top). The blot was then stripped and reprobed with anti-BLNK antibodies (bottom) (54a). (B) Cells were left unstimulated (U) or were stimulated through the indicated chimeras (C) for 2 min. Lysates were precipitated with GST-BLNK-SH2. The samples were immunoblotted with antiphosphotyrosine antibodies (top). The blot was stripped and reprobed with anti-Igα antibodies (bottom). (C) Cells were stimulated as for panel B. Lysates were immunoprecipitated (IP) with anti-PDGFRβ antibodies, divided into three equal aliquots, and far-Western blotted with GST-BLNK-SH2 (top) or GST (middle) followed by anti-GST antibodies or Western blotted with antiphosphotyrosine antibodies (bottom). (D) Cells were stimulated as for panel B for the times indicated. Lysates were immunoprecipitated with anti-PDGFRβ antibodies, divided into two equal aliquots, and Western blotted with antiphosphotyrosine antibodies (top) and far-Western blotted with the GST-BLNK-SH2 fusion protein followed by anti-GST antibodies (bottom).
FIG. 5.
Fusion of BLNK to Igα176,204 rescues signaling. (A) Diagram and expression of PDGFRβ/Igα176,204/BLNK. Full-length BLNK was fused to the Igα176,204 construct with a six-alanine linker inserted between Igα and BLNK. Whole-cell lysates were prepared from 2 × 106 cells expressing the Igα176,204/BLNK or wild-type Igα chimeras and immunoblotted with anti-BLNK (left) and anti-PDGFRβ (right) antisera. PDGFRβ/Igα migrates at approximately 130 kDa, and PDGFRβ/Igα176,204/BLNK migrates at 205 kDa. (B) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated chimeras (C) for 1 min. Lysates were immunoprecipitated with anti-PLC-γ2 antibodies and immunoblotted with antiphosphotyrosine (PTyr) antibodies (top). The blot was then stripped and reprobed with anti-PLC-γ2 antibodies (bottom). (C) Cells were loaded with Fura red and fluo-3 and stimulated either through the BCR (left) or the indicated chimeras (right). The relative mean increase in intracellular calcium is plotted as a function of time. Left arrows, addition of stimulating antibody; right arrows addition of ionomycin. The PDGF-BB ligand was added at the same time as the stimulating antibodies. (D) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated chimeras (C) for 5 min. Lysates were immunoblotted with anti-phospho-ERK1 and -ERK2 antibodies (top). The blot was then stripped and reprobed with anti-ERK1 and -ERK2 antibodies (bottom). (E) Cells were stimulated as for panel D. Lysates were precipitated with GST-c-Jun and subjected to a kinase assay and SDS-PAGE, followed by detection of phosphorylated c-Jun by autoradiography.
FIG. 6.
BLNK binds to endogenous phospho-Igα. (A) Cells were left unstimulated (U) or were stimulated through the BCR (B) for 2 min. Lysates were precipitated with anti-Igβ antibodies, GST, or GST-BLNK-SH2 and immunoblotted with antiphosphotyrosine (anti-PTyr) antibodies (top). The blots were then stripped and reprobed with anti-Igα antibodies (bottom). ∗, position of the BLNK-SH2 fusion protein; ∗∗, position of Igα. (B) Samples were stimulated as for panel A. Lysates were immunoprecipitated (IP) with control Ig (cIg) antibodies, antiphosphotyrosine antibodies, or anti-Igβ antibodies, and each was then divided into three equal aliquots. Immunoprecipitations were blotted with antiphosphotyrosine antibodies (left) or the GST-BLNK-SH2 fusion protein (middle) or GST (right) followed by anti-GST antibodies. (C) Samples were stimulated as for panel A for the times indicated. Lysates were immunoprecipitated with anti-Igβ antibodies and divided into two equal aliquots. Immunoprecipitations were blotted with antiphosphotyrosine antibodies (top) or the GST-BLNK-SH2 fusion protein followed by anti-GST antibodies (bottom). (D) A20IIA1.6 cells were left unstimulated (U) or were stimulated through the BCR (B) for 2 min. Lysates were precipitated with cIg or anti-Igβ antibodies and analyzed by immunoblotting with antiphosphotyrosine antibodies (left). In a parallel experiment, lysates were immunoprecipitated with anti-Igβ antibodies and immunoblotted with anti-BLNK antibodies (right). Longer exposures of the same blots illustrating the interaction of BLNK with the unstimulated BCR are also presented (bottom). Arrows, migratory position of BLNK. (E) (Left) J558Lμm cells were left untransfected (J558Lμm) or transfected by means of a retrovirus with either wild-type, full-length Igα (J558Lμm/wt Igα) or full-length Igα containing a Y204F mutation (J558Lμm/IgαY204F) and stimulated through the BCR with anti-IgM antibodies (5 × 106 cells/lane). Lysates were precipitated with GST-BLNK-SH2, and associated phosphorylated proteins were detected by antiphosphotyrosine blotting. (Right) Lysates of each cell type (5 × 106 cells) were subjected to anti-Igα immunoprecipitations followed by anti-Igα Western blotting to confirm equal Igα expression in the two transfectants. (F) WEHI-231 cells were labeled with 32PO4 and then stimulated through the BCR for 5 min. Anti-Igα immunoprecipitates were resolved by reducing or nonreducing 2D SDS-PAGE. Excised bands were digested with V8 protease, and eluted peptides were resolved by TLE and then TLC (left). To identify each peptide, a synthetic peptide corresponding to the entire cytoplasmic tail of Igα was phosphorylated with baculovirus-produced Lck and digested with V8 protease and subjected to TLE and TLC (right). Elution of each spot and sequencing using tandem mass spectrometry revealed the listed sequences. O, origin; Y*, phosphotyrosine.
FIG. 7.
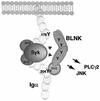
Model of Syk and BLNK cooperation in mediating PLC-γ2 and JNK activation. Upon BCR stimulation Syk is recruited to, and activated by, the phosphorylated ITAM tyrosines in the Igα cytoplasmic tail. Subsequent or concurrent recruitment of BLNK to phospho-Y204, through its SH2 domain, brings it into proximity to Syk. We postulate that the secondary, phosphotyrosine-independent association site at Y176 orients the tyrosines of BLNK to the kinase domain of Syk, facilitating BLNK phosphorylation and linkage to downstream pathways.
Similar articles
- BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells.
Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan AC, Kurosaki T. Ishiai M, et al. Immunity. 1999 Jan;10(1):117-25. doi: 10.1016/s1074-7613(00)80012-6. Immunity. 1999. PMID: 10023776 - Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-alpha.
Engels N, Wollscheid B, Wienands J. Engels N, et al. Eur J Immunol. 2001 Jul;31(7):2126-34. doi: 10.1002/1521-4141(200107)31:7<2126::aid-immu2126>3.0.co;2-o. Eur J Immunol. 2001. PMID: 11449366 - BLNK: connecting Syk and Btk to calcium signals.
Kurosaki T, Tsukada S. Kurosaki T, et al. Immunity. 2000 Jan;12(1):1-5. doi: 10.1016/s1074-7613(00)80153-3. Immunity. 2000. PMID: 10661400 Review. No abstract available. - Cbl-b positively regulates Btk-mediated activation of phospholipase C-gamma2 in B cells.
Yasuda T, Tezuka T, Maeda A, Inazu T, Yamanashi Y, Gu H, Kurosaki T, Yamamoto T. Yasuda T, et al. J Exp Med. 2002 Jul 1;196(1):51-63. doi: 10.1084/jem.20020068. J Exp Med. 2002. PMID: 12093870 Free PMC article. - Multitasking of Ig-alpha and Ig-beta to regulate B cell antigen receptor function.
Wienands J, Engels N. Wienands J, et al. Int Rev Immunol. 2001;20(6):679-96. doi: 10.3109/08830180109045585. Int Rev Immunol. 2001. PMID: 11913945 Review.
Cited by
- The immunoglobulin tail tyrosine motif upgrades memory-type BCRs by incorporating a Grb2-Btk signalling module.
Engels N, König LM, Schulze W, Radtke D, Vanshylla K, Lutz J, Winkler TH, Nitschke L, Wienands J. Engels N, et al. Nat Commun. 2014 Nov 21;5:5456. doi: 10.1038/ncomms6456. Nat Commun. 2014. PMID: 25413232 Free PMC article. - B cell receptor accessory molecule CD79α: characterisation and expression analysis in a cartilaginous fish, the spiny dogfish (Squalus acanthias).
Li R, Wang T, Bird S, Zou J, Dooley H, Secombes CJ. Li R, et al. Fish Shellfish Immunol. 2013 Jun;34(6):1404-15. doi: 10.1016/j.fsi.2013.02.015. Epub 2013 Feb 28. Fish Shellfish Immunol. 2013. PMID: 23454429 Free PMC article. - A transcriptome-based approach to identify functional modules within and across primary human immune cells.
Mola S, Foisy S, Boucher G, Major F, Beauchamp C, Karaky M, Goyette P, Lesage S, Rioux JD. Mola S, et al. PLoS One. 2020 May 29;15(5):e0233543. doi: 10.1371/journal.pone.0233543. eCollection 2020. PLoS One. 2020. PMID: 32469933 Free PMC article. - Targeting B-cell receptor signaling in leukemia and lymphoma: how and why?
Allen JC, Talab F, Slupsky JR. Allen JC, et al. Int J Hematol Oncol. 2016 May;5(1):37-53. doi: 10.2217/ijh-2016-0003. Epub 2016 May 26. Int J Hematol Oncol. 2016. PMID: 30302202 Free PMC article. Review. - Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells.
O'Neill SK, Veselits ML, Zhang M, Labno C, Cao Y, Finnegan A, Uccellini M, Alegre ML, Cambier JC, Clark MR. O'Neill SK, et al. Proc Natl Acad Sci U S A. 2009 Apr 14;106(15):6262-7. doi: 10.1073/pnas.0812922106. Epub 2009 Mar 30. Proc Natl Acad Sci U S A. 2009. PMID: 19332776 Free PMC article.
References
- Adachi, T., J. Wienands, C. Wakabayashi, H. Yakura, M. Reth, and T. Tsubata. 2001. SHP-1 requires inhibitory co-receptors to down-modulate B cell antigen receptor-mediated phosphorylation of cellular substrates. J. Biol. Chem. 276:26648-26655. - PubMed
- Alberola-Ila, J., S. Takaki, J. D. Kerner, and R. M. Perlmutter. 1997. Differential signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 15:125-154. - PubMed
- Bork, P., and B. Margolis. 1995. A phosphotyrosine interaction domain. Cell 80:693-694. - PubMed
- Burg, D. L., M. T. Furlong, M. L. Harrison, and R. L. Geahlen. 1994. Interactions of Lyn with the antigen receptor during B cell activation. J. Biol. Chem. 269:28136-28142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM052736/GM/NIGMS NIH HHS/United States
- GM52736/GM/NIGMS NIH HHS/United States
- HL07065/HL/NHLBI NIH HHS/United States
- AI42787/AI/NIAID NIH HHS/United States
- GM56187/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous