Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest - PubMed (original) (raw)
Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest
Anuradha S Budhu et al. Mol Cell Biol. 2002 Apr.
Abstract
Cellular retinoic acid-binding protein II (CRABP-II) is an intracellular lipid-binding protein that associates with retinoic acid with a subnanomolar affinity. We previously showed that CRABP-II enhances the transcriptional activity of the nuclear receptor with which it shares a common ligand, namely, the retinoic acid receptor (RAR), and we suggested that it may act by delivering retinoic acid to this receptor. Here, the mechanisms underlying the effects of CRABP-II on the transcriptional activity of RAR and the functional consequences of these effects were studied. We show that CRABP-II, a predominantly cytosolic protein, massively undergoes nuclear localization upon binding of retinoic acid; that it interacts with RAR in a ligand-dependent fashion; and that, in the presence of retinoic acid, the CRABP-II-RAR complex is a short-lived intermediate. The data establish that potentiation of the transcriptional activity of RAR stems directly from the ability of CRABP-II to channel retinoic acid to the receptor. We demonstrate further that overexpression of CRABP-II in MCF-7 mammary carcinoma cells dramatically enhances their sensitivity to retinoic acid-induced growth inhibition. Conversely, diminished expression of CRABP-II renders these cells retinoic acid resistant. Taken together, the data unequivocally establish the function of CRABP-II in modulating the RAR-mediated biological activities of retinoic acid.
Figures
FIG. 1.
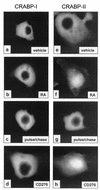
GFP-tagged CRABP-II, but not CRABP-I, localizes to the nucleus in response to ligand. COS-7 cells were transfected with GFP-CRABP-I (a to d) or GFP-CRABP-II (e to h). Images of GFP-tagged proteins were collected following a 3-h incubation with the ligands. Treatments were as follows: panels a and e, DMSO (vehicle); b and f, 1 μM RA; c and g, cells treated with 1 μM RA for 3 h followed by a 3-h chase with fresh media devoid of ligand (RA pulse/chase); d and h, 1 μM CD270.
FIG. 2.
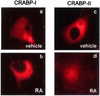
Endogenous CRABP-II, but not CRABP-I, localizes to the nucleus in response to ligand. COS-7 cells were treated with DMSO (vehicle; a and c) or 1 μM RA (b and d) for 3 h. Cells were immunostained for CRABP-I (a and b) or CRABP-II (c and d) as detailed in Materials and Methods.
FIG. 3.
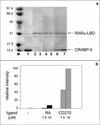
CRABP-II stably interacts with RARα-LBD in the presence of CD270. Coprecipitation assays were conducted as described in Materials and Methods. (a) Proteins were resolved by SDS-PAGE and visualized by Coomassie blue staining. Arrows indicate the positions of CRABP-II and RARα-LBD. Lanes: M, molecular weight marker; 1, CRABP-II at 50% of total input; 2, RARα-LBD immobilized on Ni+2-NTA-agarose beads; 3, immobilized RARα-LBD incubated with CRABP-II in the absence of ligand; 4 and 5, immobilized RARα-LBD incubated with CRABP-II in the presence of 7.5 and 18.75 μM RA, respectively; 6 and 7, immobilized RARα-LBD incubated with CRABP-II in the presence of 7.5 and 18.75 μM CD270, respectively. (b) Quantitation of bands shown in Fig. 2a.
FIG. 4.
CRABP-II does not enhance the transcriptional activity of RAR in the presence of CD270. Cells were cotransfected with a luciferase reporter construct driven by a RAR responsive element, a pCH110 vector (internal standard), and an empty expression vector, or an expression vector for either CRABP-I or CRABP-II. Cells were treated with RA (20 nM), CD270 (100 nM), or a combination of the two ligands for 24 h. Luciferase activity normalized to the activity of β-galactosidase is shown. Data are means ± standard errors of the mean (SEM; n = 3).
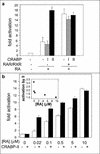
FIG. 5.
CRABP-II enhances the transcriptional activity of RAR in COS-7 cells only under limiting cellular levels of RAR or RA. Cells were cotransfected with a luciferase reporter construct driven by a RAR responsive element, a pCH110 vector (internal standard), and an empty expression vector or an expression vector for either CRABP-I or CRABP-II. Cells were treated with RA for 24 h and luciferase activity was measured and normalized for β-galactosidase activity. Data are presented as fold induction relative to cells treated with vehicle alone. Bars represent means ± SEM (n = 3). (a) The effect of CRABP-II on RA-induced reporter gene expression in the presence of endogenous retinoid receptors, or upon ectopic coexpression of hRXRα and hRARα. (b) The effect of CRABP-II on RA-induced reporter gene expression at various RA concentrations. The inset to panel b depicts the fold activation observed upon cotransfection of CRABP-II relative to fold activation in the absence of ectopic binding protein at the various RA concentrations.
FIG. 6.
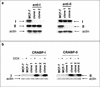
MCF-7 derivatives that stably under- or overexpress CRABP. (a) Western blot analyses of parental MCF-7 cells (MCF-7) and of their derivatives expressing antisense constructs for either CRABP-I or CRABP-II (anti-I and anti-II). Fifty micrograms of total cell lysate protein was loaded in each lane, and blots were also probed for actin to ensure equivalent loading. Data from two independent clones are shown. (b) Western blot analyses of MCF-7 lines conditionally overexpressing either CRABP-I or CRABP-II. Cells were assayed in the absence of doxycycline or following treatment with 1 μg of doxycycline/ml. Ten micrograms of total cell lysate protein was loaded in each lane, and blots were probed for actin to ensure equivalent loading. Data from two independent clones are shown.
FIG. 7.
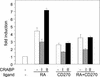
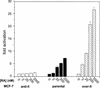
CRABP-II enhances the transcriptional activity of RAR in MCF-7 cells. Transactivation assays were carried out in parental MCF-7 cells, in MCF-7 clones that stably express CRABP-II antisense (anti-II), or in cells that stably overexpress CRABP-II (over-II). Cells were cotransfected with a luciferase reporter construct driven by a RAR responsive element along with a pCH110 vector (internal standard). Cells were treated with the denoted concentrations of RA for 24 h, and luciferase activity was measured and normalized for β-galactosidase activity. Data are presented as fold induction relative to cells treated with vehicle alone. Data are means ± SEM (n = 3).
FIG. 8.
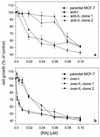
CRABP-II sensitizes MCF-7 cells to RA-induced growth inhibition. Parental MCF-7 cells and their derivatives that display minimized expression of CRABP-I (anti-I) or CRABP-II (anti-II) (a) or that overexpress CRABP-I (over-I) or CRABP-II (over-II) (b) were treated with RA for 5 days. To induce overexpression of CRABP, cells were also treated with doxycycline. Viable cell number was scored with the MTT assay as described in Materials and Methods. Data shown represent cell growth as a percent of vehicle-treated cell growth (control) and are the mean ± SEM (n = 5).
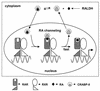
FIG. 9.
A model for the mechanism of action of CRABP-II. CRABP-II is predominantly cytosolic in the absence of ligand. Upon ligand binding, holo-CRABP-II relocates into the nucleus where it associates with apo-RAR to form a complex that mediates direct channeling of RA and facilitates the ligation of the receptor. The ligand-depleted CRABP-II rapidly dissociates from holo-RAR, releasing the receptor to its transcriptional activities. RALDH, retinal dehydrogenase.
Similar articles
- Localization of the RAR interaction domain of cellular retinoic acid binding protein-II.
Budhu A, Gillilan R, Noy N. Budhu A, et al. J Mol Biol. 2001 Jan 26;305(4):939-49. doi: 10.1006/jmbi.2000.4340. J Mol Biol. 2001. PMID: 11162104 - Nuclear translocation of cellular retinoic acid-binding protein II is regulated by retinoic acid-controlled SUMOylation.
Majumdar A, Petrescu AD, Xiong Y, Noy N. Majumdar A, et al. J Biol Chem. 2011 Dec 9;286(49):42749-42757. doi: 10.1074/jbc.M111.293464. Epub 2011 Oct 13. J Biol Chem. 2011. PMID: 21998312 Free PMC article. - Cellular retinoic acid-binding protein II: a coactivator of the transactivation by the retinoic acid receptor complex RAR.RXR.
Wolf G. Wolf G. Nutr Rev. 2000 May;58(5):151-3. doi: 10.1111/j.1753-4887.2000.tb01851.x. Nutr Rev. 2000. PMID: 10860396 Review. - The cellular retinoic acid binding proteins.
Donovan M, Olofsson B, Gustafson AL, Dencker L, Eriksson U. Donovan M, et al. J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):459-65. doi: 10.1016/0960-0760(95)00092-e. J Steroid Biochem Mol Biol. 1995. PMID: 7626495 Review.
Cited by
- [Expressions and significances of CRABPII and E-FABP in non-small cell lung cancer].
Liu Q, Wang S, Xu H, Zhang S. Liu Q, et al. Zhongguo Fei Ai Za Zhi. 2013 Jan;16(1):12-9. doi: 10.3779/j.issn.1009-3419.2013.01.03. Zhongguo Fei Ai Za Zhi. 2013. PMID: 23327868 Free PMC article. Chinese. - Crosstalk between lysine-specific demethylase 1 (LSD1) and histone deacetylases mediates antineoplastic efficacy of HDAC inhibitors in human breast cancer cells.
Vasilatos SN, Katz TA, Oesterreich S, Wan Y, Davidson NE, Huang Y. Vasilatos SN, et al. Carcinogenesis. 2013 Jun;34(6):1196-207. doi: 10.1093/carcin/bgt033. Epub 2013 Jan 25. Carcinogenesis. 2013. PMID: 23354309 Free PMC article. - Role of retinoid signaling in the regulation of spermatogenesis.
Chung SS, Wolgemuth DJ. Chung SS, et al. Cytogenet Genome Res. 2004;105(2-4):189-202. doi: 10.1159/000078189. Cytogenet Genome Res. 2004. PMID: 15237207 Free PMC article. Review. - Structural analysis of site-directed mutants of cellular retinoic acid-binding protein II addresses the relationship between structural integrity and ligand binding.
Vaezeslami S, Jia X, Vasileiou C, Borhan B, Geiger JH. Vaezeslami S, et al. Acta Crystallogr D Biol Crystallogr. 2008 Dec;64(Pt 12):1228-39. doi: 10.1107/S0907444908032216. Epub 2008 Nov 18. Acta Crystallogr D Biol Crystallogr. 2008. PMID: 19018099 Free PMC article. - Sterol-dependent nuclear import of ORP1S promotes LXR regulated trans-activation of apoE.
Lee S, Wang PY, Jeong Y, Mangelsdorf DJ, Anderson RG, Michaely P. Lee S, et al. Exp Cell Res. 2012 Oct 1;318(16):2128-42. doi: 10.1016/j.yexcr.2012.06.012. Epub 2012 Jun 20. Exp Cell Res. 2012. PMID: 22728266 Free PMC article.
References
- Boylan, J. F., and L. J. Gudas. 1992. The level of CRABP-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem cells. J. Biol. Chem. 267:21486-21491. - PubMed
- Bucco, R. A., W. L. Zheng, J. T. Davis, E. Sierra-Rivera, K. G. Osteen, A. K. Chaudhary, and D. E. Ong. 1997. Cellular retinoic acid-binding protein (II) presence in rat uterine epithelial cells correlates with their synthesis of retinoic acid. Biochemistry 36:4009-4014. - PubMed
- Budhu, A., R. Gillilan, and N. Noy. 2001. Localization of the RAR interaction domain of cellular retinoic acid binding protein-II. J. Mol. Biol. 305:939-949. - PubMed
- Chambon, P. 1996. A decade of molecular biology of retinoic acid receptors. FASEB J. 10:940-954. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous