Linkage and association of the glutamate receptor 6 gene with autism - PubMed (original) (raw)
Linkage and association of the glutamate receptor 6 gene with autism
S Jamain et al. Mol Psychiatry. 2002.
Abstract
A genome scan was previously performed and pointed to chromosome 6q21 as a candidate region for autism. This region contains the glutamate receptor 6 (GluR6 or GRIK2) gene, a functional candidate for the syndrome. Glutamate is the principal excitatory neurotransmitter in the brain and is directly involved in cognitive functions such as memory and learning. We used two different approaches, the affected sib-pair (ASP) method and the transmission disequilibrium test (TDT), to investigate the linkage and association between GluR6 and autism. The ASP method, conducted with additional markers on the 51 original families and in eight new sibling pairs, showed a significant excess of allele sharing, generating an elevated multipoint maximum LOD score (ASPEX MLS = 3.28). TDT analysis, performed in the ASP families and in an independent data set of 107 parent-offspring trios, indicated a significant maternal transmission disequilibrium (TDTall P = 0.0004). Furthermore, TDT analysis (with only one affected proband per family) showed significant association between GluR6 and autism (TDT association P = 0.008). In contrast to maternal transmission, paternal transmission of GluR6 alleles was as expected in the absence of linkage, suggesting a maternal effect such as imprinting. Mutation screening was performed in 33 affected individuals, revealing several nucleotide polymorphisms (SNPs), including one amino acid change (M867I) in a highly conserved domain of the intracytoplasmic C-terminal region of the protein. This change is found in 8% of the autistic subjects and in 4% of the control population and seems to be more maternally transmitted than expected to autistic males (P = 0.007). Taken together, these data suggest that GluR6 is in linkage disequilibrium with autism.
Figures
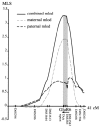
Figure 1
Maternal, paternal and combined LOD scores at each marker along the chromosome 6q21 region calculated by ASPEX.
Figure 2
Structure of the human GluR6 gene. a. Genomic organisation of the human GluR6 gene. Exon sequences were identified by BLAST analysis, using the human GluR6 cDNA sequence (NM_021956) and three genomic sequences (AP002528, AP002529, AP002530); b. Predicted exon-intron dsRNA around the Q/R editing site (intron 12 containing the ECS) of GluR6 pre-mRNA by the RNA mfold software. The nucleotides of intron 12 omitted from presentation are indicated in the loop.
Figure 3
Sequence analysis and isoforms of the human GluR6 transcript, a. Location of all SNPs identified in this study, b. Conservation of the Methionine 867 in different species and location of the protein kinase C (PKC) phosphorylation consensus site; c. RT-PCR performed on human hippocampus (h), amygdala (a) and whole brain (wb) RNA; forward primers were chosen in exon 15, 15bis or 15ter; the reverse primer is the same for all PCRs and is located in exon 16. d. C-terminal protein sequences of the different GluR6 isoforms.
Similar articles
- Glutamate receptor 6 gene (GluR6 or GRIK2) polymorphisms in the Indian population: a genetic association study on autism spectrum disorder.
Dutta S, Das S, Guhathakurta S, Sen B, Sinha S, Chatterjee A, Ghosh S, Ahmed S, Ghosh S, Usha R. Dutta S, et al. Cell Mol Neurobiol. 2007 Dec;27(8):1035-47. doi: 10.1007/s10571-007-9193-6. Epub 2007 Aug 22. Cell Mol Neurobiol. 2007. PMID: 17712621 Free PMC article. - Family-based association study between autism and glutamate receptor 6 gene in Chinese Han trios.
Shuang M, Liu J, Jia MX, Yang JZ, Wu SP, Gong XH, Ling YS, Ruan Y, Yang XL, Zhang D. Shuang M, et al. Am J Med Genet B Neuropsychiatr Genet. 2004 Nov 15;131B(1):48-50. doi: 10.1002/ajmg.b.30025. Am J Med Genet B Neuropsychiatr Genet. 2004. PMID: 15389769 - Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism.
Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, Buxbaum JD. Ramoz N, et al. Am J Psychiatry. 2004 Apr;161(4):662-9. doi: 10.1176/appi.ajp.161.4.662. Am J Psychiatry. 2004. PMID: 15056512 - Maternal transmission disequilibrium of the glutamate receptor GRIK2 in schizophrenia.
Bah J, Quach H, Ebstein RP, Segman RH, Melke J, Jamain S, Rietschel M, Modai I, Kanas K, Karni O, Lerer B, Gourion D, Krebs MO, Etain B, Schürhoff F, Szöke A, Leboyer M, Bourgeron T. Bah J, et al. Neuroreport. 2004 Aug 26;15(12):1987-91. doi: 10.1097/00001756-200408260-00031. Neuroreport. 2004. PMID: 15305151 - The genetics of autism.
Muhle R, Trentacoste SV, Rapin I. Muhle R, et al. Pediatrics. 2004 May;113(5):e472-86. doi: 10.1542/peds.113.5.e472. Pediatrics. 2004. PMID: 15121991 Review.
Cited by
- Structure, Function, and Regulation of the Kainate Receptor.
Dhingra S, Yadav J, Kumar J. Dhingra S, et al. Subcell Biochem. 2022;99:317-350. doi: 10.1007/978-3-031-00793-4_10. Subcell Biochem. 2022. PMID: 36151381 Review. - Prospective MEG biomarkers in ASD: pre-clinical evidence and clinical promise of electrophysiological signatures.
Port RG, Anwar AR, Ku M, Carlson GC, Siegel SJ, Roberts TP. Port RG, et al. Yale J Biol Med. 2015 Mar 4;88(1):25-36. eCollection 2015 Mar. Yale J Biol Med. 2015. PMID: 25745372 Free PMC article. Review. - Polymorphisms of Ionotropic Glutamate Receptor-Related Genes and the Risk of Autism Spectrum Disorder in a Chinese Population.
Xie X, Hou F, Li L, Chen Y, Liu L, Luo X, Gu H, Li X, Zhang J, Gong J, Song R. Xie X, et al. Psychiatry Investig. 2019 May;16(5):379-385. doi: 10.30773/pi.2019.02.26.3. Epub 2019 May 23. Psychiatry Investig. 2019. PMID: 31132842 Free PMC article. - Trafficking of kainate receptors.
Pahl S, Tapken D, Haering SC, Hollmann M. Pahl S, et al. Membranes (Basel). 2014 Aug 20;4(3):565-95. doi: 10.3390/membranes4030565. Membranes (Basel). 2014. PMID: 25141211 Free PMC article. Review. - Metabotropic glutamate receptor genetic variants and peripheral receptor expression affects trait scores of autistic probands.
Dutta N, Chatterjee M, Saha S, Sinha S, Mukhopadhyay K. Dutta N, et al. Sci Rep. 2024 Apr 12;14(1):8558. doi: 10.1038/s41598-024-59290-2. Sci Rep. 2024. PMID: 38609494 Free PMC article.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington DC: 1994.
- Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. - PubMed
- Gillberg C, Wing L. Autism: not an extremely rare disorder. Acta Psychiatr Scand. 1999;99:399–406. - PubMed
- Miles JH, Hillman RE. Value of a clinical morphology examination in autism. Am J Med Genet. 2000;91:245–253. - PubMed
- Ritvo ER, Freeman BJ, Pingree C, Mason-Brothers A, Jorde L, Jenson WR, et al. The UCLA-University of Utah epidemiologic survey of autism: prevalence. Am J Psychiatry. 1989;146:194–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources