Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus - PubMed (original) (raw)
Comparative Study
Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus
Thomas Klausberger et al. J Neurosci. 2002.
Abstract
Networks of parvalbumin (PV)-expressing basket cells are implicated in synchronizing cortical neurons at various frequencies, through GABA(A) receptor-mediated synaptic action. These cells are interconnected by GABAergic synapses and gap junctions, and converge with a different class of cholecystokinin-expressing, PV-negative basket cells onto pyramidal cells. To define the molecular specializations in the synapses of the two basket cell populations, we used quantitative electron microscopic immunogold localization of GABA(A) receptors. Synapses formed by PV-positive basket cells on the somata of pyramidal cells had several-fold higher density of alpha1 subunit-containing receptors than synapses made by PV-negative basket cells, most of which were immunonegative. The density of the beta2/3 subunits was similar in the two populations of synapse, indicating similar overall receptor density. Synapses interconnecting parvalbumin-expressing basket cells contained a 3.6 times higher overall density of GABA(A) receptor (beta2/3 subunits) and 3.2 times higher density of alpha1 subunit labeling compared with synapses formed by boutons of PV-positive basket cells on pyramidal cells. Thus, PV-positive basket cells mainly act through alpha1 subunit-containing GABA(A) receptors, but the receptor density depends on the postsynaptic cell type. These observations, together with previously reported enrichment of the alpha2 subunit-containing receptors in synapses made by PV-negative basket cells, indicate that the number and subtypes of GABA(A) receptors present in different synapse populations are regulated by both presynaptic and postsynaptic influences. The high number of GABA(A) receptors in synapses on basket cells might contribute to the precisely timed phasing of basket cell activity.
Figures
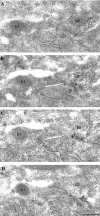
Fig. 1.
Differential immunolabeling for the α1 subunit of the GABAA receptor in synapses (open arrows) formed by PV-negative (b1) or PV-positive (b2) boutons on a pyramidal cell soma.A, Electron micrograph of a section immunolabeled for PV (10 nm gold particles; small arrows) showing an immunopositive (b2, small arrows) and an immunonegative (b1) bouton converging on the same pyramidal cell body. B–D, Three sections serial to A are immunolabeled for the α1subunit (silver-intensified ultra small gold particles;arrowheads). The synapse made by the PV-positive, but not the one made by the PV-negative, bouton is consistently labeled (arrowheads) for the α1 subunit. Scale bar: A–D, 0.2 μm.
Fig. 2.
Strong immunolabeling for the α1 subunit of the GABAA receptor in synapses made by PV-positive boutons on a PV-positive interneuron soma in the pyramidal cell layer. A, Electron micrograph of a section immunolabeled for PV (10 nm gold particles; small arrows). Three synaptic boutons (b), as well as the soma of the interneuron, are PV positive. The rightmost bouton is connected to the cell body via three punctae adherentiae. B–E, Four sections serial to A are immunolabeled for the α1 subunit (silver-intensified ultra small gold particles; arrowheads), demonstrating consistent and strong immunolabeling in the synapses made by all three boutons. Scale bar: A–E, 0.2 μm.
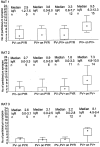
Fig. 3.
Differences in immunoreactivity for the α1 subunit of the GABAA receptor in synapses made by PV-negative or PV-positive boutons on pyramidal cell somata (PYR) and PV-positive boutons on PV-positive interneuron somata in three adult rats. Immunoreactivity is measured as density values (number of gold particles per length of synaptic junction) obtained from one to five serial sections of each synaptic membrane. Small squares, rectangles, and_bars_ indicate median, interquartile range (IqR), and minimum–maximum values, respectively. The three synapse populations were different from each other in all combinations and in all three rats (Kruskal–Wallis test,p < 0.001; post hoc Dunn test,p < 0.05). Note that the overall level of immunoreactivity was different in the three rats, but differences among synapse populations were comparable.
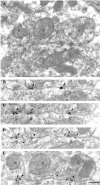
Fig. 4.
Similar immunolabeling for the β2/3subunits of the GABAA receptor in synapses (open arrows) formed by PV-negative (b1) or PV-positive (b2) boutons on a pyramidal cell soma.A, B, Electron micrographs showing two serial sections coimmunolabeled for β2/3 subunits (10 nm gold particles; arrowheads) and for PV (5 nm gold particles; small arrows). The synaptic junction of each bouton comes into the section plane in different sections. Scale bar:A, B, 0.2 μm.
Fig. 5.
Immunolabeling for the β2/3 subunits of the GABAA receptor in a synapse (open arrows) on the soma of a PV-positive interneuron in the pyramidal cell layer. A–C, Electron micrographs showing three serial sections coimmunolabeled for the β2/3subunits (10 nm gold particles; arrowheads) and for PV (5 nm gold particles; small arrows). Note that the bouton (b), as well as the soma of the interneuron, is PV positive (small arrows). Scale bar:A–C, 0.2 μm.
Fig. 6.
Difference in immunoreactivity for the β2/3 subunits of the GABAA receptor on PV-negative, PV-positive, or pooled populations of synapses on pyramidal cell somata (PYR), and PV-positive synapses on PV-positive interneuron somata in three adult rats. Immunoreactivity is measured as density values (number of gold particles per length of synaptic junction) obtained from one to five serial sections of each synaptic membrane. Small squares,rectangles, and bars indicate median, interquartile range (IqR), and minimum–maximum values, respectively. Immunoreactivity for the β2/3 subunits in synapses made by PV-negative and PV-positive boutons on pyramidal cell somata was not different in any of the rats (Mann–Whitney_U_ test, p > 0.5) (Nyiri et al., 2001); therefore, the data were pooled. Synapses on pyramidal cell somata and PV-positive synapses on interneurons were different in all three rats (Mann–Whitney U test, p< 0.005).
Similar articles
- Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat.
Nyíri G, Freund TF, Somogyi P. Nyíri G, et al. Eur J Neurosci. 2001 Feb;13(3):428-42. doi: 10.1046/j.1460-9568.2001.01407.x. Eur J Neurosci. 2001. PMID: 11168550 - The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus.
Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. Somogyi P, et al. Neuropharmacology. 1996;35(9-10):1425-44. doi: 10.1016/s0028-3908(96)00086-x. Neuropharmacology. 1996. PMID: 9014159 - Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling.
Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Kasugai Y, et al. Eur J Neurosci. 2010 Dec;32(11):1868-88. doi: 10.1111/j.1460-9568.2010.07473.x. Epub 2010 Nov 14. Eur J Neurosci. 2010. PMID: 21073549 Free PMC article. - Cell type specificity of GABA(A) receptor mediated signaling in the hippocampus.
Semyanov A. Semyanov A. Curr Drug Targets CNS Neurol Disord. 2003 Aug;2(4):240-7. doi: 10.2174/1568007033482832. Curr Drug Targets CNS Neurol Disord. 2003. PMID: 12871034 Review. - Cortical basket cell dysfunction in schizophrenia.
Curley AA, Lewis DA. Curley AA, et al. J Physiol. 2012 Feb 15;590(4):715-24. doi: 10.1113/jphysiol.2011.224659. Epub 2012 Jan 4. J Physiol. 2012. PMID: 22219337 Free PMC article. Review.
Cited by
- Distribution and postnatal development of chondroitin sulfate proteoglycans in the perineuronal nets of cholinergic motoneurons innervating extraocular muscles.
Ritok A, Kiss P, Zaher A, Wolf E, Ducza L, Bacskai T, Matesz C, Gaal B. Ritok A, et al. Sci Rep. 2022 Dec 14;12(1):21606. doi: 10.1038/s41598-022-25692-3. Sci Rep. 2022. PMID: 36517521 Free PMC article. - Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia.
Beneyto M, Abbott A, Hashimoto T, Lewis DA. Beneyto M, et al. Cereb Cortex. 2011 May;21(5):999-1011. doi: 10.1093/cercor/bhq169. Epub 2010 Sep 15. Cereb Cortex. 2011. PMID: 20843900 Free PMC article. - ErbB4 reduces synaptic GABAA currents independent of its receptor tyrosine kinase activity.
Mitchell RM, Janssen MJ, Karavanova I, Vullhorst D, Furth K, Makusky A, Markey SP, Buonanno A. Mitchell RM, et al. Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19603-8. doi: 10.1073/pnas.1312791110. Epub 2013 Nov 11. Proc Natl Acad Sci U S A. 2013. PMID: 24218551 Free PMC article. - Distribution of N-Acetylgalactosamine-Positive Perineuronal Nets in the Macaque Brain: Anatomy and Implications.
Mueller AL, Davis A, Sovich S, Carlson SS, Robinson FR. Mueller AL, et al. Neural Plast. 2016;2016:6021428. doi: 10.1155/2016/6021428. Epub 2016 Jan 3. Neural Plast. 2016. PMID: 26881119 Free PMC article. - Distance-dependent inhibition facilitates focality of gamma oscillations in the dentate gyrus.
Strüber M, Sauer JF, Jonas P, Bartos M. Strüber M, et al. Nat Commun. 2017 Oct 2;8(1):758. doi: 10.1038/s41467-017-00936-3. Nat Commun. 2017. PMID: 28970502 Free PMC article.
References
- Aika Y, Ren JQ, Kosaka K, Kosaka T. Quantitative analysis of GABA-like-immunoreactive and parvalbumin-containing neurons in the CA1 region of the rat hippocampus using a stereological method, the disector. Exp Brain Res. 1994;99:267–276. - PubMed
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. - PubMed
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous