Lack of collagen XVIII/endostatin results in eye abnormalities - PubMed (original) (raw)
Lack of collagen XVIII/endostatin results in eye abnormalities
Naomi Fukai et al. EMBO J. 2002.
Abstract
Mice lacking collagen XVIII and its proteolytically derived product endostatin show delayed regression of blood vessels in the vitreous along the surface of the retina after birth and lack of or abnormal outgrowth of retinal vessels. This suggests that collagen XVIII/endostatin is critical for normal blood vessel formation in the eye. All basement membranes in wild-type eyes, except Descemet's membrane, showed immunogold labeling with antibodies against collagen XVIII. Labeling at sites where collagen fibrils in the vitreous are connected with the inner limiting membrane and separation of the vitreal matrix from the inner limiting membrane in mutant mice indicate that collagen XVIII is important for anchoring vitreal collagen fibrils to the inner limiting membrane. The findings provide an explanation for high myopia, vitreoretinal degeneration and retinal detachment seen in patients with Knobloch syndrome caused by loss-of-function mutations in collagen XVIII.
Figures
Fig. 1. (A) Diagram showing domain structure of α1(XVIII) collagen (top) and exon structure of central portion of Col18a1 gene (bottom). Size markers [in 100 amino acid (AA) and 1 kb units] are shown at right. Triple-helical domains are indicated by open boxes, and non-triple helical protein domains by a heavy line. Non-triple helical domains at carboxyl and amino ends are labeled NC1 and NC11, respectively. Exons are represented by vertical bars and introns by a line. A unique _Sal_I site (S), in exon 30, corresponds to site within COL5 domain, used for insertion of _neo_R cassette in targeting construct. (B) Diagram showing targeting construct (middle) and its relationship to wild-type gene (top) and targeted gene following homologous recombination (bottom). Open arrow indicates the direction of transcription of PKG/Neo gene. Probe KE was used for screening ES cell clones. Southern blotting with this probe detects a 19 kb _Eco_RI fragment in wild-type DNA and a 6 kb _Eco_RI fragment in targeted DNA. Primers (SW, SN and RW) and their directions (arrowheads) used for PCR-based genotyping are shown at the bottom. E, _Eco_RI; S, _Sal_I; K, _Kpn_I; N, _Not_I. (C) Gel electrophoresis of PCR products with mouse tail DNA and primer pairs SW/RW and SN/RW. Lane 1, size markers; lanes 2 and 5, DNA from homozygous Col18a1 null mice; lanes 3, 4, 6 and 7, DNA from heterozygous mice; lanes 8 and 9, DNA from wild-type mice. Sizes of PCR products indicated on the left. (D) Northern blot probed for Col18a1 with RNA from wild-type, heterozygous and homozygous mutant mice. The probe was from 3′ untranslated and endostatin region of mRNA. Lanes 1, 4, 5 and 6, RNA from homozygous Col18a1 null mice; lane 2, RNA from heterozygous Col18a1+/– mouse; lanes 3 and 7, RNA from wild-type mice. In lanes 1–4 RNA from adult liver; in lane 5 from adult lung; in lane 6 from adult kidney; in lane 7 from embryonic day 17.5 whole embryos. Results of reprobing the blot for Gapdh are shown at the bottom. Positions of 18S and 28S RNAs are indicated on the left.
Fig. 2. Delayed regression of hyaloid capillaries in _Col18a1_–/– mice compared with age-matched wild-type mice at postnatal days 0.5 (A, B), 4 (C, D), 8 (E, F), 16 (G, H) and 24 (I, J). Abbreviations: L, lens; V, vitreous; R, retina. Symbols: black arrows, hyaloid capillaries; white arrows, TVL; asterisk, contact between hyaloid capillary and retina. Original magnifications: (A–F), (I) and (J), ×40; (G) and (H), ×80; all insets, ×160. Horizontal sections through eye of wild-type (A) and _Col18a1_–/– mouse (B) at postnatal day 0.5 showing transverse sections of hyaloid capillaries at internal surface of retina and vessel branches attached to lens capsule (TVL). Insets: few vessels attached to surface of retina in wild-type mice, but were seen more frequently in _Col18a1_–/– mice. Hyaloid capillaries are clearly dissociated from retinal surface and start to show signs of regression in wild-type mice at day 4 (C). Hyaloid capillaries still near retina in _Col18a1_–/– mice (D) or in contact with it [(D), inset]. Hyaloid capillaries have disappeared from vitreous (E) or some rudiments can be seen in the vicinity of the lens [(E), inset] in wild-type eyes at postnatal day 8. In age-matched _Col18a1_–/– mice, hyaloid capillaries are found within vitreous (F), near or attached to retina [(F), inset]. In some _Col18a1_–/– capillaries the diameter is increased and numerous erythrocytes [(F), inset] are seen. Hyaloid capillaries were completely removed from vitreous in wild-type mice at day 16 (G). In _Col18a1_–/– mice, hyaloid vessels still present in vitreous (H). Some persistent vessels are still connected to retina [(H), inset]. No hyaloid capillaries were found in 24-day-old wild-type mice (I). Most sections from _Col18a1_–/– eyes are comparable to wild-type samples; however, some sections show numerous persistent and enlarged vessels in vitreous (J).
Fig. 3. The number of hyaloid vessel profiles in vitreous (A) and TVL (B) at different days in sections from wild-type (open squares, WT) and homozygous _Col18a1_–/– null (filled circles) mice. At each time point means of data from eight wild-type and nine _Col18a1_–/– mice are shown. Standard errors of the mean are indicated by vertical bars; significant differences between wild-type and knockout values are indicated by triple asterisks.
Fig. 4. Blood vessels in retinas of 4-day-old mice. Whole-mount immunofluorescence with antibodies against collagen IV in wild-type (A) and _Col18a1_–/– (B–D) mice, showing irregular development of blood vessels from optic nerve head (asterisks). In some _Col18a1_–/– samples there are no developing vessels (B), in some cases irregular growth of retinal capillaries (C), and in some, samples denser growth of capillaries (D) when compared with wild-type samples (A). Original magnification ×120.
Fig. 5. Expression of VEGF by in situ hybridization in retinal neuroglia of collagen XVIII null [–/– (A and B)] and wild-type [+/+ (C and D)] eyes. Rectangular areas in (A) and (C) are shown at a higher magnification in (B) and (D). Relative levels of VEGF (±SD, n = 4) by quantitative PCR shown in (B) and (D). C, cornea; L, lens; I, inner cell layer; O, outer cell layer. Scale bars: (B), 200 µm; (D), 50 µm.
Fig. 6. Staining of wild-type mouse eye at postnatal day 1 with anti-collagen XVIII antibodies. Positive staining of basement membranes in TVL, around lens (L) and in VHP along inner limiting membrane (ILM). Scale bar, 200 µm.
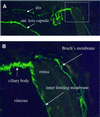
Fig. 7. Immunofluorescence staining of anterior portion of adult wild-type mouse eye with anti-collagen XVIII antibodies. Basement membranes of iris and anterior lens capsule are indicated in (A). Rectangular area in (A), shown at a higher magnification in (B), shows staining of basement membrane of ciliary body, inner limiting membrane and Bruch’s membrane. Original magnification: (A), ×100; (B), ×400.
Fig. 8. Labeling of ocular and epidermal basement membranes of wild-type mice with gold-labeled anti-collagen XVIII antibodies. (A) Clusters of gold particles on matrix side (with collagen fibrils) along lamina densa (asterisk) of Bowman’s membrane (scale bar 200 nm). Occasional clusters also on epithelial side. (B) Labeling of lamina densa (asterisk) along epidermal basement membrane (scale bar 400 nm); occasional larger particles, which are products of post-labeling enhancement, are visible. (C) Labeling of lamina densa (asterisk) of inner limiting membrane (scale bar 200 nm). Arrows indicate clusters of gold particles at sites where vitreal collagen fibrils approach inner limiting membrane. These particles are clearly evident in (D) (scale bar 50 nm), which shows the rectangular area in (C) at a higher magnification.
Fig. 9. Immunolabeling of Bruch’s membrane with gold-labeled antibodies against collagen XVIII. (A) Low magnification overview (scale bar 400 nm) shows pigment epithelium in upper left-hand corner, capillary of choriocapillaris at lower right, and clusters of gold particles along lamina densa (asterisks) of epithelial and endothelial basement membranes; label is also seen associated with collagen fibrils in the space between the two basement membranes. (B) Tangential section through Bruch’s membrane shown at a higher magnification (scale bar 250 nm). Clusters of gold particles seen on each side of lamina densa (asterisk) of pigment epithelial cells in upper left-hand corner. At the lower right, clusters of particles seen associated with collagen fibrils. Microfibrils and associated electron-dense material (probably elastin) in central area show little or no labeling.
Fig. 10. Ultrastructure of retinal surface and adjacent vitreous in adult wild-type [+/+, (A)] and collagen XVIII null [–/–, (B)] mice. Lamina densa of inner limiting membrane is indicated by asterisks. Arrows, vitreal collagen fibrils in wild-type eye; N, nuclei of retinal cells. Scale bar, 1000 nm.
Fig. 11. Growth of B16F10 melanoma and T241 fibrosarcoma tumors in wild-type (open symbols) and collagen XVIII null (filled symbols) mice. Vertical bars represent standard deviations in tumor volume.
Similar articles
- Age-dependent iris abnormalities in collagen XVIII/endostatin deficient mice with similarities to human pigment dispersion syndrome.
Marneros AG, Olsen BR. Marneros AG, et al. Invest Ophthalmol Vis Sci. 2003 Jun;44(6):2367-72. doi: 10.1167/iovs.02-1180. Invest Ophthalmol Vis Sci. 2003. PMID: 12766032 - Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment.
Takahashi K, Saishin Y, Saishin Y, Silva RL, Oshima Y, Oshima S, Melia M, Paszkiet B, Zerby D, Kadan MJ, Liau G, Kaleko M, Connelly S, Luo T, Campochiaro PA. Takahashi K, et al. FASEB J. 2003 May;17(8):896-8. doi: 10.1096/fj.02-0824fje. Epub 2003 Mar 28. FASEB J. 2003. PMID: 12670875 - Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity.
Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Sasaki T, et al. J Mol Biol. 2000 Sep 1;301(5):1179-90. doi: 10.1006/jmbi.2000.3996. J Mol Biol. 2000. PMID: 10966814 - Physiological role of collagen XVIII and endostatin.
Marneros AG, Olsen BR. Marneros AG, et al. FASEB J. 2005 May;19(7):716-28. doi: 10.1096/fj.04-2134rev. FASEB J. 2005. PMID: 15857886 Review. - Collagen XVIII/endostatin structure and functional role in angiogenesis.
Zatterstrom UK, Felbor U, Fukai N, Olsen BR. Zatterstrom UK, et al. Cell Struct Funct. 2000 Apr;25(2):97-101. doi: 10.1247/csf.25.97. Cell Struct Funct. 2000. PMID: 10885579 Review.
Cited by
- Positive selection rather than relaxation of functional constraint drives the evolution of vision during chicken domestication.
Wang MS, Zhang RW, Su LY, Li Y, Peng MS, Liu HQ, Zeng L, Irwin DM, Du JL, Yao YG, Wu DD, Zhang YP. Wang MS, et al. Cell Res. 2016 May;26(5):556-73. doi: 10.1038/cr.2016.44. Epub 2016 Apr 1. Cell Res. 2016. PMID: 27033669 Free PMC article. - Protein composition and biomechanical properties of in vivo-derived basement membranes.
Halfter W, Candiello J, Hu H, Zhang P, Schreiber E, Balasubramani M. Halfter W, et al. Cell Adh Migr. 2013 Jan-Feb;7(1):64-71. doi: 10.4161/cam.22479. Epub 2012 Nov 15. Cell Adh Migr. 2013. PMID: 23154404 Free PMC article. Review. - Esophageal muscle physiology and morphogenesis require assembly of a collagen XIX-rich basement membrane zone.
Sumiyoshi H, Mor N, Lee SY, Doty S, Henderson S, Tanaka S, Yoshioka H, Rattan S, Ramirez F. Sumiyoshi H, et al. J Cell Biol. 2004 Aug 16;166(4):591-600. doi: 10.1083/jcb.200402054. Epub 2004 Aug 9. J Cell Biol. 2004. PMID: 15302855 Free PMC article. - Developmental and pathogenic mechanisms of basement membrane assembly.
Yurchenco PD, Patton BL. Yurchenco PD, et al. Curr Pharm Des. 2009;15(12):1277-94. doi: 10.2174/138161209787846766. Curr Pharm Des. 2009. PMID: 19355968 Free PMC article. Review. - Micromorphology analysis of the anterior human lens capsule.
Ţălu Ş, Sueiras VM, Moy VT, Ziebarth NM. Ţălu Ş, et al. Mol Vis. 2018 Dec 31;24:902-912. eCollection 2018. Mol Vis. 2018. PMID: 30713427 Free PMC article.
References
- Alon T., Hemo,I., Itin,A., Pe’er,J., Stone,J. and Keshet,E. (1995) Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature Med., 1, 1024–1028. - PubMed
- Benjamin L.E., Hemo,I. and Keshet,E. (1998) A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development, 125, 1591–1598. - PubMed
- Bischoff P.M., Wajer,S.D. and Flower,R.W. (1983) Scanning electron microscopic studies of the hyaloid vascular system in newborn mice exposed to O2 and CO2. Graefes Arch. Clin. Exp. Ophthalmol., 220, 257–263. - PubMed
- Böhme K., Li,Y., Oh,S.P. and Olsen,B.R. (1995) Primary structure of the long and short splice variants of mouse collagen XII and their tissue-specific expression during embryonic development. Dev. Dyn., 204, 432–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases