Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation - PubMed (original) (raw)
Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation
Ulla E Petäjä-Repo et al. EMBO J. 2002.
Abstract
The endoplasmic reticulum (ER) is recognized as an important site for regulating cell surface expression of membrane proteins. We recently reported that only a fraction of newly synthesized delta opioid receptors could leave the ER and reach the cell surface, the rest being degraded by proteasomes. Here, we demonstrate that membrane-permeable opioid ligands facilitate maturation and ER export of the receptor, thus acting as pharmacological chaperones. We propose that these ligands stabilize the newly synthesized receptor in the native or intermediate state of its folding pathway, possibly by inducing stabilizing conformational constrains within the hydrophobic core of the protein. The receptor precursors that are retained in the ER thus represent fully competent folding intermediates that can be targets for pharmacological intervention aimed at regulating receptor expression and cellular responsiveness. The pharmacological chaperone action is independent of the intrinsic signaling efficacy of the ligand, since both agonists and antagonists were found to promote receptor maturation. This novel property of G protein-coupled receptor ligands may have important implications when considering their effects on cellular responsiveness during therapeutic treatments.
Figures
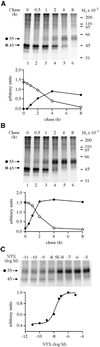
Fig. 1. HδOR synthesis and maturation in untreated and NTX-treated HEK-293S-hδOR-FLAG cells. Cells were labeled for 60 min with 150 µCi/ml of [35S]methionine/cysteine and then chased in medium supplemented with 5 mM methionine in the absence (A) or presence (B) of 10 µM NTX for the indicated times. Alternatively, the cells were chased for 4 h in the presence of NTX at the indicated final concentration (C). Cellular membranes were isolated and solubilized in DDM and the receptors immunoprecipitated using the immobilized anti-FLAG M2 antibody as described in Materials and methods. The samples were analyzed by SDS–PAGE and fluorography. Molecular weights of the markers used to calibrate the gels are indicated on the right. The different receptor forms are indicated by arrows and symbols (filled circle, _M_r 55 000; open circle, _M_r 45 000). The graphs in (A) and (B) describe the time course of appearance and disappearance of the _M_r 45 000 and _M_r 55 000 receptor species. Intensities of these species were obtained by densitometric scanning and the values were normalized to the maximum labeling of the mature receptor at 4 h of chase in untreated cells. In (C), the intensity of the mature hδOR species (_M_r 55 000) was normalized to the maximum labeling of that species and the data were fitted to a sigmoidal dose response equation using the GraphPad Prism program version 2.01.
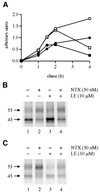
Fig. 2. The inability of a membrane-impermeable opioid ligand to block or mimic the effect of NTX on hδOR maturation. HEK-293S-hδOR-FLAG cells were labeled with [35S]methionine/cysteine and then chased for the indicated times in the absence (filled circles) or presence of 50 nM NTX (open circles), 10 µM LE (filled squares) or 50 nM NTX + 10 µM LE (open squares). Cellular membranes were solubilized and the receptors immunoprecipitated and analyzed as described in Figure 1. The graph in (A) describes the time course of appearance and disappearance of the mature _M_r 55 000 receptor. Intensity of this species was obtained by densitometric scanning and the values were normalized to the maximum labeling at 4 h of chase in untreated cells. The fluorographs in (B) and (C) correspond to the samples at 2 and 4 h of chase, respectively.
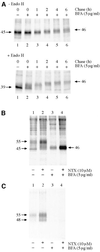
Fig. 3. HδOR synthesis and maturation in BFA-treated HEK-293S-hδOR-FLAG cells. Cells were pulse-labeled with [35S]methionine/ cysteine and chased for the indicated times (A) or for 6 h (B, C). BFA (5 µg/ml) was added to the medium 60 min prior to labeling and NTX (10 µM) at the beginning of the chase. In (A), receptors were immunoprecipitated as described in Figure 1 and incubated for 16 h at 37°C in the absence or presence of 25 mU/ml of Endo H as indicated. In (B) and (C), cell surface proteins were biotinylated by incubating the intact metabolically labeled cells with sulfo-NHS-biotin (0.5 mg/ml) and the cellular membranes were isolated and solubilized in DDM. Receptors from one-quarter of the extract were immunoprecipitated using the immobilized anti-FLAG M2 antibody to isolate the total pool of labeled receptors (B). The rest of the extract was used to isolate the cell surface receptors by sequential precipitations using the immobilized streptavidin and anti-FLAG M2 antibody (C). The purified receptors were analyzed by SDS–PAGE and fluorography.
Fig. 4. Rescue of ER-retained hδOR precursors by NTX treatment. HEK-293S-hδOR-FLAG cells were pulse-labeled with [35S]methionine/cysteine and chased for 4 h in the absence (lane 1) or presence of 10 µM NTX (lanes 2–5). NTX was added to the chase medium at the beginning of the chase (lane 5), or at 1 h (lane 4), 2 h (lane 3) or 3 h (lane 2) after the beginning of the chase. Receptors were then isolated by immunoprecipitation and analyzed as described in Figure 1.
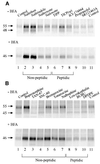
Fig. 5. Both membrane-permeable opioid antagonists and agonists can function as pharmacological chaperones. HEK-293S-hδOR-FLAG cells were pulse-labeled with [35S]methionine/cysteine and chased for 6 h in the presence of 10 µM of the indicated opioid antagonists (A) or agonists (B). BFA (5 µg/ml) or the vehicle (–BFA) was added to the medium 60 min prior to labeling. Receptors were then isolated by immunoprecipitation and analyzed as described in Figure 1. The abbreviations used are: BUBU, Tyr-D-Ser[-_O_-C(CH3)3]-Gly-Phe-Leu-Thr-_O_-C(CH3)3; DPDPE, cyclic[D-Pen2,D-Pen5]enkephalin; ICI-174864, N,_N_-diallyl-Tyr-Aib-Aib-Phe-Leu-OH; SNC-80, (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,_N_-diethylbenzamidine; SR121463A, 1-[4-(_N_-_tert_-butylcarbamoyl)-2- methoxybenzenesulfonyl]-5-ethoxy-3-spiro-[4-(2-morpholinoethoxy)cyclohexane]indol-2-one, fumerate; TAN-67, 2-methyl-4aα-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12aα-octahydroquinolino-[2,3,3-g]isoquinoline); TICP(ψ), Tyr-Ticψ[CH2NH]Cha-Phe-OH; TIPP, Tyr-Tic-Phe-Phe-OH.
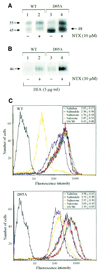
Fig. 6. Rescue of the cMyc-tagged wild-type hδOR and the D95A mutant by pharmacological chaperones. (A) Western blot analysis of the steady-state levels of the wild-type hδOR and the D95A mutant in stably transfected HEK-293S cells treated or not with 10 µM NTX for 24 h. Immunoblotting was carried out using the polyclonal anti-cMyc antibody following immunoprecipitation of the receptor with the immobilized monoclonal anti-cMyc antibody. (B) Maturation of the wild-type hδOR and the D95A mutant in BFA-treated cells. Stably transfected HEK-293S cells were pulse-labeled with [35S]methionine/cysteine and chased for 6 h in the absence (lane 1 and 3) or presence (lanes 2 and 4) of 10 µM NTX. BFA (5 µg/ml) was added to the medium 60 min prior to labeling. Receptors were isolated by immunoprecipitation using the immobilized monoclonal anti-cMyc antibody and analyzed as in Figure 1. (C) FACS analysis of the cell surface receptors. The HEK-293S cells stably expressing either the cMyc-tagged wild-type hδOR (upper panel) or the D95A mutant (lower panel) were treated for 24 h in the absence or presence of 10 µM of the following opioid ligands: naltriben (green), naltrindole (red), NTX (orange), naloxone (blue) or SNC-80 (yellow). The cell surface receptors were labeled with the monoclonal anti-cMyc antibody and quantitated by FACS as described in Materials and methods. The purple curve represents untreated cells; the background signal obtained in the absence of the primary antibody is shown in black. The values presented in the insets indicate the fold increase in the mean cell surface fluorescence promoted by each ligand and represent the mean ± SE of four independent experiments.
Similar articles
- Opioid receptor pharmacological chaperones act by binding and stabilizing newly synthesized receptors in the endoplasmic reticulum.
Leskelä TT, Markkanen PM, Pietilä EM, Tuusa JT, Petäjä-Repo UE. Leskelä TT, et al. J Biol Chem. 2007 Aug 10;282(32):23171-83. doi: 10.1074/jbc.M610896200. Epub 2007 Jun 5. J Biol Chem. 2007. PMID: 17550902 - The near-death experience of delta opioid receptors leads to new drug targets.
Bartlett SE, Whistler JL. Bartlett SE, et al. Mol Interv. 2002 Jun;2(3):134-6. doi: 10.1124/mi.2.3.134. Mol Interv. 2002. PMID: 14993372 No abstract available. - Specific ligands as pharmacological chaperones: The transport of misfolded G-protein coupled receptors to the cell surface.
Nakamura M, Yasuda D, Hirota N, Shimizu T. Nakamura M, et al. IUBMB Life. 2010 Jun;62(6):453-9. doi: 10.1002/iub.344. IUBMB Life. 2010. PMID: 20503438 Review. - Receptor-associated protein (RAP): a specialized chaperone for endocytic receptors.
Willnow TE. Willnow TE. Biol Chem. 1998 Aug-Sep;379(8-9):1025-31. Biol Chem. 1998. PMID: 9792434 Review.
Cited by
- Regulation of G protein-coupled receptor export trafficking.
Dong C, Filipeanu CM, Duvernay MT, Wu G. Dong C, et al. Biochim Biophys Acta. 2007 Apr;1768(4):853-70. doi: 10.1016/j.bbamem.2006.09.008. Epub 2006 Sep 23. Biochim Biophys Acta. 2007. PMID: 17074298 Free PMC article. Review. - Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice.
Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Morinville A, et al. J Neurosci. 2003 Jun 15;23(12):4888-98. doi: 10.1523/JNEUROSCI.23-12-04888.2003. J Neurosci. 2003. PMID: 12832511 Free PMC article. - Protein quality control: the who's who, the where's and therapeutic escapes.
Roth J, Yam GH, Fan J, Hirano K, Gaplovska-Kysela K, Le Fourn V, Guhl B, Santimaria R, Torossi T, Ziak M, Zuber C. Roth J, et al. Histochem Cell Biol. 2008 Feb;129(2):163-77. doi: 10.1007/s00418-007-0366-7. Epub 2007 Dec 13. Histochem Cell Biol. 2008. PMID: 18075753 Free PMC article. Review. - Pharmacological chaperoning: a primer on mechanism and pharmacology.
Leidenheimer NJ, Ryder KG. Leidenheimer NJ, et al. Pharmacol Res. 2014 May;83:10-9. doi: 10.1016/j.phrs.2014.01.005. Epub 2014 Feb 14. Pharmacol Res. 2014. PMID: 24530489 Free PMC article. Review. - Increased plasma membrane expression of human follicle-stimulating hormone receptor by a small molecule thienopyr(im)idine.
Janovick JA, Maya-Núñez G, Ulloa-Aguirre A, Huhtaniemi IT, Dias JA, Verbost P, Conn PM. Janovick JA, et al. Mol Cell Endocrinol. 2009 Jan 27;298(1-2):84-8. doi: 10.1016/j.mce.2008.09.015. Epub 2008 Sep 20. Mol Cell Endocrinol. 2009. PMID: 18848862 Free PMC article.
References
- Alewijnse A.E., Timmerman,H., Jacobs,E.H., Smit,M.J., Roovers,E., Cotecchia,S. and Leurs,R. (2000) The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H2 receptor. Mol. Pharmacol., 57, 890–898. - PubMed
- Aridor M. and Balch,W.E. (1999) Integration of endoplasmic reticulum signaling in health and disease. Nature Med., 5, 745–751. - PubMed
- Bermak J.C., Li,M., Bullock,C. and Zhou,Q.-Y. (2001) Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nature Cell Biol., 3, 492–498. - PubMed
- Bot G., Blake,A.D., Li,S. and Reisine,T. (1998) Mutagenesis of the mouse delta opioid receptor converts (–)-buprenorphine from a partial agonist to an antagonist. J. Pharmacol. Exp. Ther., 284, 283–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources