A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(-)CD4(+) CD1d-dependent precursor stage - PubMed (original) (raw)
A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(-)CD4(+) CD1d-dependent precursor stage
Daniel G Pellicci et al. J Exp Med. 2002.
Abstract
The development of CD1d-dependent natural killer T (NKT) cells is poorly understood. We have used both CD1d/alpha-galactosylceramide (CD1d/alphaGC) tetramers and anti-NK1.1 to investigate NKT cell development in vitro and in vivo. Confirming the thymus-dependence of these cells, we show that CD1d/alphaGC tetramer-binding NKT cells, including NK1.1(+) and NK1.1(-) subsets, develop in fetal thymus organ culture (FTOC) and are completely absent in nude mice. Ontogenically, CD1d/alphaGC tetramer-binding NKT cells first appear in the thymus, at day 5 after birth, as CD4(+)CD8(-)NK1.1(-)cells. NK1.1(+) NKT cells, including CD4(+) and CD4(-)CD8(-) subsets, appeared at days 7-8 but remained a minor subset until at least 3 wk of age. Using intrathymic transfer experiments, CD4(+)NK1.1(-) NKT cells gave rise to NK1.1(+) NKT cells (including CD4(+) and CD4(-) subsets), but not vice-versa. This maturation step was not required for NKT cells to migrate to other tissues, as NK1.1(-) NKT cells were detected in liver and spleen as early as day 8 after birth, and the majority of NKT cells among recent thymic emigrants (RTE) were NK1.1(-). Further elucidation of this NKT cell developmental pathway should prove to be invaluable for studying the mechanisms that regulate the development of these cells.
Figures
Figure 1.
CD1d-dependent NKT cell development is thymus dependent. (A) Thymocytes were harvested from FTOC after 11, 13, 15, and 18 d of culture and stained for multi-parameter flow cytometric analysis. An adult thymus sample is included as a labeling control. The first column shows CD1d/αGC tetramer+αβTCR+ cells with the percentages indicated. The second column shows CD4 versus NK1.1 expression on NKT cells, gated as shown in the first column. The third column shows CD4 versus CD8 expression on NKT cells, gated as shown in the first column. (B) The data from all FTOC experiments were graphed and means ± standard error plotted as shown. In some cases where the error was very low, no bars are visible. Results are from 1–5 different experiments with at least four separate cultures per experiment. Tetramer, CD1d/αGC tetramer.
Figure 2.
NKT cells are absent from spleen and liver of nude mice. Spleens and livers were harvested from BALB/c nude (nu/nu) mice at day 13 after birth and at 11 wk of age. Cells were counted and labeled for flow cytometric analysis. Day 13 heterozygous (+/nu) littermates and 11-wk-old wild-type (+/+) BALB/c spleen and liver cells were examined in parallel as a control to show that labeling for CD1d/αGC tetramer+ αβTCR+ cells was as expected. Dotplots are representative of five day 13 and three 11-wk-old BALB/c nude (nu/nu) mice. Three nude mice were also screened at 7 wk of age with similar results (not shown).
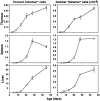
Figure 3.
Ontogeny of NKT cells. Thymuses, spleens, and livers were removed from C57BL/6 mice at various ages and harvested cells counted and labeled for flow cytometric analysis. The first column shows mean percentages of CD1d/αGC tetramer+ αβTCR+ NKT cells, and the second column shows mean numbers of these cells. The error bars represent standard error of the mean. In some cases where the error was very low, no bars are visible. Results are from 4–12 mice per time point.
Figure 4.
Phenotype of NKT cells during development. C57BL/6 mice were killed at various ages and (A) thymus, (B) spleen, and (C) liver harvested, cells counted, and labeled for flow cytometric analysis. The first column shows CD1d/αGC tetramer+αβTCR+ cells with the percentages indicated. The second column shows CD4 versus NK1.1 expression on NKT cells, gated as shown in the first column. The third column shows CD4 versus CD8 expression on NKT cells, gated as shown in the first column. As a staining control, CD4 versus CD8 labeling on total thymocytes is shown in the inset dotplot in the bottom row (Adult) of the third column. Dotplots are representative of 4–12 mice per time point. ND, not determined.
Figure 5.
Preferential thymic emigration of NK1.1− NKT cells. RTE were tracked following intrathymic injection of FITC dye. 36 h after injection, lymphocytes were harvested from thymus, spleen, and liver, counted, and stained for flow cytometric analysis. FITC+ cells were gated above the line shown in the first column. The second column shows that FITC+ (FL-1+) events in the spleen and liver do not occur when FITC has not been injected intrathymically. The third column shows CD1d/αGC tetramer+ αβTCR+ NKT cells within the RTE population. The third and fourth columns show NK1.1 expression on NKT cells within the FITC+ RTE and FITC− resident cell populations in each tissue. Dotplots are representative of four separate mice from one experiment.
Figure 6.
NK1.1− NKT cells are precursors of NK1.1**+** NKT cells. NK1.1−CD4+HSAlow and NK1.1+CD4+HSAlow populations from 4-wk-old C57BL/6 (CD45.2+) thymuses were purified by FACS® sorting and intrathymically injected into CD45.1 congenic C57BL/6 recipient mice. 1 wk later, recipient thymuses were harvested and donor-derived cells identified based on CD45.2 expression. A gate was placed around CD1d/αGC tetramer+ CD45.2+ NKT cells which were examined for CD4 versus NK1.1 expression. The percentage of acquisition-gated CD45.2+ cells that were CD1d/αGC tetramer+ is shown. These results are representative of three experiments with 1–3 recipient mice per group per experiment. Controls included transfer of CD4+HSA−/low cells that had previously been depleted of CD1d/αGC tetramer+ cells (third row) and PBS-injected thymuses (last row). Tetramer, CD1d/αGC tetramer.
Similar articles
- A subset of NKT cells that lacks the NK1.1 marker, expresses CD1d molecules, and autopresents the alpha-galactosylceramide antigen.
Hameg A, Apostolou I, Leite-De-Moraes M, Gombert JM, Garcia C, Koezuka Y, Bach JF, Herbelin A. Hameg A, et al. J Immunol. 2000 Nov 1;165(9):4917-26. doi: 10.4049/jimmunol.165.9.4917. J Immunol. 2000. PMID: 11046017 - CD1d-restricted NKT cells: an interstrain comparison.
Hammond KJ, Pellicci DG, Poulton LD, Naidenko OV, Scalzo AA, Baxter AG, Godfrey DI. Hammond KJ, et al. J Immunol. 2001 Aug 1;167(3):1164-73. doi: 10.4049/jimmunol.167.3.1164. J Immunol. 2001. PMID: 11466330 - Natural killer T-cell populations in C57BL/6 and NK1.1 congenic BALB.NK mice-a novel thymic subset defined in BALB.NK mice.
Stenström M, Sköld M, Andersson A, Cardell SL. Stenström M, et al. Immunology. 2005 Mar;114(3):336-45. doi: 10.1111/j.1365-2567.2004.02111.x. Immunology. 2005. PMID: 15720435 Free PMC article. - Mouse NK1+ T cells.
Bendelac A. Bendelac A. Curr Opin Immunol. 1995 Jun;7(3):367-74. doi: 10.1016/0952-7915(95)80112-x. Curr Opin Immunol. 1995. PMID: 7546402 Review. - Thymus medulla fosters generation of natural Treg cells, invariant γδ T cells, and invariant NKT cells: what we learn from intrathymic migration.
Cowan JE, Jenkinson WE, Anderson G. Cowan JE, et al. Eur J Immunol. 2015 Mar;45(3):652-60. doi: 10.1002/eji.201445108. Epub 2015 Feb 13. Eur J Immunol. 2015. PMID: 25615828 Free PMC article. Review.
Cited by
- ATP-binding cassette transporter G1 intrinsically regulates invariant NKT cell development.
Sag D, Wingender G, Nowyhed H, Wu R, Gebre AK, Parks JS, Kronenberg M, Hedrick CC. Sag D, et al. J Immunol. 2012 Dec 1;189(11):5129-38. doi: 10.4049/jimmunol.1201570. Epub 2012 Oct 24. J Immunol. 2012. PMID: 23100511 Free PMC article. - Potent neutralizing anti-CD1d antibody reduces lung cytokine release in primate asthma model.
Nambiar J, Clarke AW, Shim D, Mabon D, Tian C, Windloch K, Buhmann C, Corazon B, Lindgren M, Pollard M, Domagala T, Poulton L, Doyle AG. Nambiar J, et al. MAbs. 2015;7(3):638-50. doi: 10.1080/19420862.2015.1016693. MAbs. 2015. PMID: 25751125 Free PMC article. - TIM-4 is expressed on invariant NKT cells but dispensable for their development and function.
Zhang X, Gu J, Zhou L, Mi QS. Zhang X, et al. Oncotarget. 2016 Nov 1;7(44):71099-71111. doi: 10.18632/oncotarget.12153. Oncotarget. 2016. PMID: 27662666 Free PMC article. - NKAP Regulates Invariant NKT Cell Proliferation and Differentiation into ROR-γt-Expressing NKT17 Cells.
Thapa P, Chen MW, McWilliams DC, Belmonte P, Constans M, Sant'Angelo DB, Shapiro VS. Thapa P, et al. J Immunol. 2016 Jun 15;196(12):4987-98. doi: 10.4049/jimmunol.1501653. Epub 2016 May 9. J Immunol. 2016. PMID: 27183586 Free PMC article. - Ligand-dependent inhibition of CD1d-restricted NKT cell development in mice transgenic for the activating receptor Ly49D.
Voyle RB, Beermann F, Lees RK, Schümann J, Zimmer J, Held W, MacDonald HR. Voyle RB, et al. J Exp Med. 2003 Apr 7;197(7):919-25. doi: 10.1084/jem.20021615. J Exp Med. 2003. PMID: 12682111 Free PMC article.
References
- Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells - development, specificity, and function. Annu. Rev. Immunol. 15:535–562. - PubMed
- Godfrey, D.I., K.J.L. Hammond, L.D. Poulton, M.J. Smyth, and A.G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today. 21:573–583. - PubMed
- Bendelac, A., N. Killeen, D.R. Littman, and R.H. Schwartz. 1994. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 263:1774–1778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials