LIS1, CLIP-170's key to the dynein/dynactin pathway - PubMed (original) (raw)
. 2002 May;22(9):3089-102.
doi: 10.1128/MCB.22.9.3089-3102.2002.
Michal Caspi, Fabrice P Cordelières, Jim P Dompierre, Denis L Dujardin, Cynthia Koifman, Patrick Martin, Casper C Hoogenraad, Anna Akhmanova, Niels Galjart, Jan R De Mey, Orly Reiner
Affiliations
- PMID: 11940666
- PMCID: PMC133759
- DOI: 10.1128/MCB.22.9.3089-3102.2002
LIS1, CLIP-170's key to the dynein/dynactin pathway
Frédéric M Coquelle et al. Mol Cell Biol. 2002 May.
Abstract
CLIP-170 is a plus-end tracking protein which may act as an anticatastrophe factor. It has been proposed to mediate the association of dynein/dynactin to microtubule (MT) plus ends, and it also binds to kinetochores in a dynein/dynactin-dependent fashion, both via its C-terminal domain. This domain contains two zinc finger motifs (proximal and distal), which are hypothesized to mediate protein-protein interactions. LIS1, a protein implicated in brain development, acts in several processes mediated by the dynein/dynactin pathway by interacting with dynein and other proteins. Here we demonstrate colocalization and direct interaction between CLIP-170 and LIS1. In mammalian cells, LIS1 recruitment to kinetochores is dynein/dynactin dependent, and recruitment there of CLIP-170 is dependent on its site of binding to LIS1, located in the distal zinc finger motif. Overexpression of CLIP-170 results in a zinc finger-dependent localization of a phospho-LIS1 isoform and dynactin to MT bundles, raising the possibility that CLIP-170 and LIS1 regulate dynein/dynactin binding to MTs. This work suggests that LIS1 is a regulated adapter between CLIP-170 and cytoplasmic dynein at sites involved in cargo-MT loading, and/or in the control of MT dynamics.
Figures
FIG. 1.
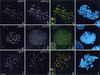
Colocalization of LIS1 and CLIP-170 at MT-dependent and MT-independent sites. HeLa cells were labeled with a polyclonal anti-LIS1 antibody (a, e, and i), the monoclonal 4D3 anti-CLIP-170 antibody (b, f, and j), and CREST autoimmune serum. Panels c, g, and k are superimpositions of CLIP-170 (red) and LIS1 (green) signals. Panels d, h, and l are superimpositions of CREST (green) and DAPI signals. LIS1 and CLIP-170 colocalize on the nuclear envelope in prophase cells (a to d). In prometaphase, they colocalize on the kinetochores and MT tips (early prometaphase) (e to h, arrows) and on the cortical sites (late prometaphase) (i to l, arrows). HeLa cells were transiently transfected with LIS1-DsRed (m) and fixed under conditions preserving the MT tips (see Materials and Methods). They were labeled with polyclonal anti-CLIP-170 antibody TA (n). Panel o is a superimposition of LIS1-DsRed (green) and CLIP-170 (red). All images are maximal-intensity projections of x/y optical section stacks, acquired by three-dimensional deconvolution microscopy. Bars, 5 μm.
FIG. 2.
Physical interaction between LIS1 and CLIP-170. (a) LIS1 interacts with the C-terminal region of CLIP-170 in a yeast two-hybrid system. Positive interactions were demonstrated between PEG-LIS1 and pJG4-5-Cter or Z1m. Other controls with empty baits or an unrelated bait (Caspar) resulted in no blue colonies. (b) CLIP-170 interaction with LIS1 requires the presence of the WD5 domain. GST pull-down assays using GST-Cter or GST (negative control) with individual domains of LIS1 that were in vitro translated in a reticulocyte lysate using [35S]methionine were performed. The percentage of input protein that was pulled down specifically by GST-Cter is indicated. The results presented are averages from four independent experiments ± standard errors of the means. (c) Representative autoradiogram used for quantification of the different LIS1 domains in panel b. (d) Recombinant six-histidine LIS1 and GST-Cter-CLIP-170 interact in a GST pull-down assay. The negative control GST does not interact with LIS1. (e) Schematic presentation of the different C-terminal CLIP-170 constructs used in this study. (f) LIS1 interacts with the C-terminal domain of CLIP-170 and requires the second zinc finger domain (results of GST pull-down assays with brain extract, reticulocyte lysate, and HeLa cell extract) are shown. (g) LIS1, CLIP-170, and dynein intermediate chain coimmunoprecipitate (IP) from cells transfected with DsRed-LIS1-FLAG using anti-FLAG antibodies. (h) LIS1 and CLIP-170 coimmunoprecipitate from E15.5 mouse brain extract with two different anti-CLIP-170 antibodies. The antibodies used were the MT domain antibodies anti-CLIP-115/170 antibody no. 2221 (32) and CLIP-170 C terminus (or tail)-specific antibodies (no. 2360), as described in Materials and Methods.
FIG. 3.
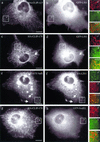
Effect of p50 overexpression on CLIP-170 and LIS1 targeting to kinetochores. Triple immunostaining of mitotic control (a to d and i to l) or EGFP-p50-overexpressing (e to h and m to p) HeLa cells for either p150 (b and f), LIS1 (c and g), and CREST (d and h) or CLIP-170 (j and n), LIS1 (k and o), and CREST (l and p). Cells were treated with 10 μM nocodazole for 4 h prior to fixation. As expected, p150 (b and f) and CLIP-170 (j and h) staining from kinetochores decreased under EGFP-p50 expression. Under these conditions LIS1 staining is also dramatically diminished (c, g, k, and o). The images in panels a and i demonstrate that there is no p50 overexpression (ox) while those in panels e and m show eGFP-p50 overexpression. All images are maximal-intensity projections of 10 consecutive planes acquired by three-dimensional deconvolution microscopy. Bar, 5 μm.
FIG. 4.
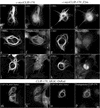
CLIP-170 localization at the kinetochore is dependent on its LIS1-binding site. COS-7 cells were transiently transfected with GFP-Cter, GFP-C2, or GFP-Z1 + 2 and blocked in prometaphase by nocodazole treatment. The fixation was preceded by extraction in Triton X-100 in order to render kinetochore-bound CLIP-170 detectable. The cells were double labeled with CREST autoimmune serum (a, e, and i) and anti-GFP (b, f, and j). Panels c, g, and k are superimpositions of CREST (red) and GFP (green) signals. Chromosomes were stained with DAPI (d, h, and l). The insets in panels c, g, and k show the relative locations of GFP chimera proteins (in green) and the CREST antigens (in red). All images are maximal-intensity projections of x/y optical section stacks acquired by three-dimensional deconvolution microscopy. Bar, 5 μm.
FIG. 5.
CLIP-170, but not CLIP-115, recruits epitope-tagged LIS1 to MT distal ends. COS-1 cells were cotransfected with HA-CLIP-115 and GFP-LIS1 (a and b), HA-CLIP-170 and GFP-LIS1 (c and d), GFP-CLIP-115(+tail) and HA-LIS1 (e and f), and HA-CLIP-170 and GFP-NudEL (g and h). In the GFP-CLIP-115(+tail) construct, the zinc “knuckle”-containing domain of CLIP-170 was transferred to CLIP-115. LIS1, recruited to CLIP-positive structures, is indicated by arrows. Panels a′ to h′ represent colored enlargements of the areas indicated in panels a to h. The merged images of panels a′ to h′ demonstrate overlap (or the lack of it) at MT distal ends of the different tagged proteins. Bar, 10 μm.
FIG. 6.
Binding of a specific phospho-LIS1 isoform to CLIP-170-induced MT bundles is dependent on the second zinc finger domain of CLIP-170. COS-7 cells were transiently transfected with c-mycCLIP-170 (a, b, e, f, i, and j), with c-mycCLIP-170-Z2m (c, d, g, h, k, and l), or with CLIP-ΔRΔC-DsRed (m, n, o, and p). The fixation was preceded by a brief extraction in Triton X-100. (a to d) The cells were double labeled with a polyclonal anti-c-myc antibody (a and c) and a polyclonal anti-LIS1 (b and d). (e to l) The cells are double labeled with the monoclonal anti-c-myc 9E10 antibody (e, g, i, and k) and the monoclonal anti-phospho-LIS1 210 antibody (f and h) or a monoclonal anti-p150 antibody (j and l). (m to o) The cells were labeled with a monoclonal anti-EB1 antibody (n) or the polyclonal antibody TA against the C-terminal portion of CLIP-170 (p). All of the CLIP-170 constructs give rise to the formation of thick MT bundles. LIS1 is always recruited to the bundles (b and d). A mutation in the second zinc finger domain (Z2m) diminishes the recruitment of a phospho-LIS1 isoform and p150 to the bundles (f and j versus h and l). Bar, 10 μm.
FIG. 7.
A model of LIS1 mediating CLIP-170 interactions with the dynein/dynactin pathway. (a) LIS1 can bind to MT bundles; however, phospho-LIS1 (recognized by the 210 antibody) binding to MT bundles is mediated through the distal zinc finger motif of CLIP-170. (b) When the distal zinc finger motif of CLIP-170 is mutated, the phospho-LIS1 isoform can no longer bind to MT bundles. This can also explain why p150/dynactin is not recruited to the MT bundles. (c) Possible kinetics of the interactions. CLIP-170 targets the dynein/dynactin complex to the MT, using phopho-LIS1 as an adapter (step 1); the motor complex, being in the proximity of the MT, associates with it (step 2); the initial static link to the MT provided by CLIP-170 is released by its hyperphosphorylation (step 3); and the retrograde dynein/dynactin-dependent movement of the cargo (endocytic vesicle, kinetochore, etc.) takes place (step 4). Tip1p, the CLIP-170 ortholog in S. pombe, has been reported by Brunner and Nurse (8) to act as an anticatastrophe factor. Based on the model depicted here, it also could be that CLIP-170 plays a similar role in eukaryotic cells by targeting MT-stabilizing factors as cargo to the plus tips. The model is based on that of Rickard and Kreis (; reviewed in references , , and 75).
Similar articles
- Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function.
Tai CY, Dujardin DL, Faulkner NE, Vallee RB. Tai CY, et al. J Cell Biol. 2002 Mar 18;156(6):959-68. doi: 10.1083/jcb.200109046. Epub 2002 Mar 11. J Cell Biol. 2002. PMID: 11889140 Free PMC article. - Colocalization of cytoplasmic dynein with dynactin and CLIP-170 at microtubule distal ends.
Vaughan KT, Tynan SH, Faulkner NE, Echeverri CJ, Vallee RB. Vaughan KT, et al. J Cell Sci. 1999 May;112 ( Pt 10):1437-47. doi: 10.1242/jcs.112.10.1437. J Cell Sci. 1999. PMID: 10212138 - Differential effects of the dynein-regulatory factor Lissencephaly-1 on processive dynein-dynactin motility.
Gutierrez PA, Ackermann BE, Vershinin M, McKenney RJ. Gutierrez PA, et al. J Biol Chem. 2017 Jul 21;292(29):12245-12255. doi: 10.1074/jbc.M117.790048. Epub 2017 Jun 2. J Biol Chem. 2017. PMID: 28576829 Free PMC article. - Regulation of dynein-dynactin-driven vesicular transport.
Liu JJ. Liu JJ. Traffic. 2017 Jun;18(6):336-347. doi: 10.1111/tra.12475. Epub 2017 Mar 28. Traffic. 2017. PMID: 28248450 Review. - LIS1 at the microtubule plus end and its role in dynein-mediated nuclear migration.
Xiang X. Xiang X. J Cell Biol. 2003 Feb 3;160(3):289-90. doi: 10.1083/jcb.200212168. J Cell Biol. 2003. PMID: 12566423 Free PMC article. Review.
Cited by
- CCSer2 gates dynein activity at the cell periphery.
Zang JL, Gibson D, Zheng AM, Shi W, Gillies JP, Stein C, Drerup CM, DeSantis ME. Zang JL, et al. bioRxiv [Preprint]. 2024 Jun 14:2024.06.13.598865. doi: 10.1101/2024.06.13.598865. bioRxiv. 2024. PMID: 38915497 Free PMC article. Preprint. - Presynaptic perspective: Axonal transport defects in neurodevelopmental disorders.
Xiong GJ, Sheng ZH. Xiong GJ, et al. J Cell Biol. 2024 Jun 3;223(6):e202401145. doi: 10.1083/jcb.202401145. Epub 2024 Apr 3. J Cell Biol. 2024. PMID: 38568173 Free PMC article. Review. - Microtubule-binding-induced allostery triggers LIS1 dissociation from dynein prior to cargo transport.
Ton WD, Wang Y, Chai P, Beauchamp-Perez C, Flint NT, Lammers LG, Xiong H, Zhang K, Markus SM. Ton WD, et al. Nat Struct Mol Biol. 2023 Sep;30(9):1365-1379. doi: 10.1038/s41594-023-01010-x. Epub 2023 Jun 15. Nat Struct Mol Biol. 2023. PMID: 37322240 Free PMC article. - LIS1 RNA-binding orchestrates the mechanosensitive properties of embryonic stem cells in AGO2-dependent and independent ways.
Kshirsagar A, Doroshev SM, Gorelik A, Olender T, Sapir T, Tsuboi D, Rosenhek-Goldian I, Malitsky S, Itkin M, Argoetti A, Mandel-Gutfreund Y, Cohen SR, Hanna JH, Ulitsky I, Kaibuchi K, Reiner O. Kshirsagar A, et al. Nat Commun. 2023 Jun 6;14(1):3293. doi: 10.1038/s41467-023-38797-8. Nat Commun. 2023. PMID: 37280197 Free PMC article. - The motor domain of the kinesin Kip2 promotes microtubule polymerization at microtubule tips.
Chen X, Portran D, Widmer LA, Stangier MM, Czub MP, Liakopoulos D, Stelling J, Steinmetz MO, Barral Y. Chen X, et al. J Cell Biol. 2023 Jul 3;222(7):e202110126. doi: 10.1083/jcb.202110126. Epub 2023 Apr 24. J Cell Biol. 2023. PMID: 37093124 Free PMC article.
References
- Akhmanova, A., C. C. Hoogenraad, K. Drabek, T. Stepanova, B. Dortland, T. Verkerk, W. Vermeulen, B. M. Burgering, C. I. De Zeeuw, F. Grosveld, and N. Galjart. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104:923-935. - PubMed
- Allan, V. 1996. Motor proteins: a dynamic duo. Curr. Biol. 6:630-633. - PubMed
- Banks, J. D., and R. Heald. 2001. Chromosome movement: dynein-out at the kinetochore. Curr. Biol. 11:R128-R31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous