The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta - PubMed (original) (raw)
The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta
Shlomit Shamash et al. J Neurosci. 2002.
Abstract
Wallerian degeneration (WD) is the inflammatory response of the nervous system to axonal injury, primarily attributable to the production of cytokines, the mediator molecules of inflammation. We presently document the involvement of the inflammatory cytokines TNFalpha, interleukin (IL)-1alpha, and IL-1beta in peripheral nerve (PNS) injury in C57/BL/6NHSD (C57/BL) mice that display the normal rapid progression of WD (rapid-WD) and C57/BL/6-WLD/OLA/NHSD mice that display abnormal slow progression of WD (slow-WD). TNFalpha and IL-1alpha mRNAs were expressed, whereas TNFalpha but not IL-1alpha protein was synthesized in intact PNS of C57/BL mice. TNFalpha and IL-1alpha protein synthesis and secretion were rapidly upregulated during rapid-WD in Schwann cells. IL-1beta mRNA expression and protein synthesis and secretion were induced sequentially in Schwann cells with a delay after injury. Thereafter, recruited macrophages contributed to the production of TNFalpha, IL-1alpha, and IL-1beta, which in turn augmented myelin phagocytosis by macrophages. Observations suggest that TNFalpha and IL-1alpha are the first cytokines with protein production that is upregulated during rapid-WD. TNFalpha and IL-1alpha may initiate, therefore, molecular and cellular events in rapid-WD (e.g., the production of additional cytokines and NGF). TNFalpha, IL-1alpha, and IL-1beta may further regulate, indirectly, macrophage recruitment, myelin removal, regeneration, and neuropathic pain. In contrast to rapid-WD, the production of TNFalpha, IL-1alpha, and IL-1beta protein was deficient in slow-WD, although their mRNAs were expressed. mRNA expression and protein production of TNFalpha, IL-1alpha, and IL-1beta were differentially regulated during rapid-WD and slow-WD, suggesting that mRNA expression, by itself, is no indication of the functional involvement of cytokines in WD.
Figures
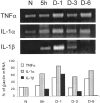
Fig. 1.
TNFα and IL-1α mRNAs but not IL-1β mRNA are detected in intact PNS of C57/BL mice (N). TNFα and IL-1α mRNAs are further detected during the first 6 d of rapid-WD studied. IL-1β mRNA is detected 5 hr and 1 and 3 d but barely at 6 d after injury during rapid-WD. Intact (N) and rapid-WD PNS segments were removed 5 hr (5h) and 1, 3, and 6 d (D-1,D-3, and D-6, respectively) after injury. The same tissues were the source for the detection of TNFα, IL-1α, IL-1β, and β-actin mRNAs by RT-PCR. RT-PCR amplification products were separated on ethidium bromide-stained 1.5% agarose gel, visualized by ultraviolet light, and photographed (top panel). The photographs were scanned, and densitometric analysis was performed. Levels of mRNA of each cytokine at each time point were further calculated as percentage of levels of β-actin mRNA in the same tissue sample (bottom panel).
Fig. 2.
TNFα protein but not IL-1α or IL-1β protein is detected in intact PNS of C57/BL mice (N). TNFα protein is further detected during the first 3 d of rapid-WD studied. IL-1α protein is first detected 5 hr after injury and thereafter during the first 3 d of rapid-WD studied. IL-1β protein is detected 1 and 3 d after injury but not 5 and 12 hr after injury. Cryostat sections of intact (N) and rapid-WD PNS segments that were removed 5 and 12 hr (_5h_and 12h, respectively) and 1 and 3 d (D-1 and D-3, respectively) after injury were used to detect TNFα, IL-1α, and IL-1β protein by immunofluorescence microscopy. Magnification: 250×.
Fig. 3.
TNFα and IL-1β are synthesized and secreted during rapid-WD. The onset of TNFα synthesis and secretion is rapid, within the first 5 hr after PNS injury. The onset of IL-1β synthesis and secretion is delayed, between 5 and 10 hr after PNS injury. PNS segments were removed from non-operated C57/BL mice and used to condition medium for 5 hr (0–5h). Similarly, PNS segments situated distal to transection sites were removed from C57/BL mice 5 hr and 1, 3, 6, and 9 d after injury and used to condition medium for 5 hr (5–10h,D-1, D-3, D-6, and_D-9_, respectively). The same conditioned media were assayed for TNFα and IL-1β content by ELISA, and production levels were calculated (picograms per milligram wet weight of tissue in 5 hr). Bars are the average of three experiments except for time point_D-3_, where six experiments were performed. In each experiment, four different PNS segments were used. Error bars indicate ±1 SEM.
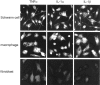
Fig. 4.
Schwann cells and recruited macrophages produce TNFα, IL-1α, and IL-1β protein. Nerve-derived fibroblasts produce low levels of TNFα but not IL-1α or IL-1β protein. Single-cell type cultures of resident Schwann cells and fibroblasts and recruited macrophages were obtained from intact, rapid-WD, and freeze-damaged PNS of C57/BL mice. The non-neuronal cells were studied for the presence of TNFα, IL-1α, and IL-1β protein by immunofluorescence microscopy. High levels of immunoreactivity for all cytokines were detected in the cytoplasm of Schwann cells and macrophages. In fibroblasts, levels of immunoreactivity to TNFα were slightly above control levels, whereas levels of immunoreactivity to IL-1α and IL-1β were the same as for control. Immunoreactivity of cytokines was localized to the cytoplasm and not cell surfaces because immunoreactivity was detected in cells permeabilized by Triton X-100 but not in non-permeabilized cells (data not shown). Magnification: 400×.
Fig. 5.
TNFα and IL-1α and IL-1β mRNA are detected in Schwann cells and macrophages. Schwann cell cultures were obtained from rapid-WD PNS of C57/BL mice, as in Figure 4, and macrophages were thioglycollate elicited. Schwann cells and macrophages were used as source for the detection of TNFα, IL-1α, IL-1β, and β-actin mRNAs by RT-PCR. RT-PCR amplification products were separated on ethidium bromide-stained 1.5% agarose gel, visualized by ultraviolet light, and photographed. The photographs were scanned, and densitometric analysis was performed. Levels of mRNA of each cytokine were further calculated as percentage of levels of β-actin mRNA in the same cell sample.
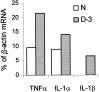
Fig. 6.
TNFα and IL-1α mRNAs but not IL-1β mRNA are detected in intact PNS of Wld mice (N). TNFα, IL-1α, and IL-1β mRNAs are detected in slow-WD domains of Wld mice 3 d after injury (D-3). Intact PNS segments and slow-WD PNS domains, 3 d after injury, were removed from Wld mice. The same tissues were the source for the detection of TNFα, IL-1α, IL-1β, and β-actin mRNAs by RT-PCR. RT-PCR amplification products were separated on ethidium bromide-stained 1.5% agarose gel, visualized by ultraviolet light, and photographed. The photographs were scanned, and densitometric analysis was performed. Levels of mRNA of each cytokine at each time point were further calculated as percentage of levels of β-actin mRNA in the same tissue sample.
Fig. 7.
TNFα, IL-1α, and IL-1β protein are detected in injury domains but not slow-WD domains of Wld mice 3 d after injury. The PNS segment, which is situated distal to the injury site, was removed 3 d after injury, and cryostat sections were taken from the injury domain and its mate slow-WD domain. These were then processed simultaneously side by side for the detection of TNFα, IL-1α, and IL-1β protein by immunofluorescence microscopy. Magnification: 250×.
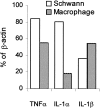
Fig. 8.
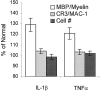
TNFα and IL-1β augment myelin phagocytosis by macrophages. Thioglycollate-elicited peritoneal macrophages were incubated in the absence or presence of TNFα (1 ng/ml) or IL-1β (0.6 ng/ml) for 36 hr. In each experiment, a macrophage population from a different mouse was studied. Thereafter, myelin phagocytosis, cell surface levels of CR3/MAC-1, and cell number (#) were quantified and further calculated as percentage of normal. Values obtained in the absence of cytokines were defined 100% normal. Bars are the average of six experiments, each performed in quadruplicate. Error bars indicate ±1 SEM.
Fig. 9.
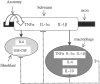
The cytokine network of Wallerian degeneration. The cellular elements depicted are a resident Schwann cell ensheathing an axon, a resident fibroblast, and a recruited macrophage.Solid lines represent induction and upregulation, and_dotted lines_ represent downregulation of the production of cytokine protein. Axotomy induces the production of TNFα and IL-1α in resident Schwann cells first. Thereafter, IL-6 and GM-CSF are produced in resident fibroblasts; then follows IL-1β production in resident Schwann cells. Macrophages, which are recruited as of the third day after injury, produce the inflammatory cytokines TNFα, IL-1α, IL-1β, and IL-6 and the anti-inflammatory cytokine IL-10. IL-10 downregulates the production of all inflammatory cytokines and itself in all non-neuronal cells. Not shown in this diagram are low, functionally insignificant levels of IL-10, which are produced by fibroblasts, and the ability of IL-6 to downregulate TNFα production (see Discussion).
Similar articles
- The cytokine network of wallerian degeneration: IL-10 and GM-CSF.
Be'eri H, Reichert F, Saada A, Rotshenker S. Be'eri H, et al. Eur J Neurosci. 1998 Aug;10(8):2707-13. Eur J Neurosci. 1998. PMID: 9767400 - Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration.
Perrin FE, Lacroix S, Avilés-Trigueros M, David S. Perrin FE, et al. Brain. 2005 Apr;128(Pt 4):854-66. doi: 10.1093/brain/awh407. Epub 2005 Feb 2. Brain. 2005. PMID: 15689362 - Interleukin 6 in intact and injured mouse peripheral nerves.
Reichert F, Levitzky R, Rotshenker S. Reichert F, et al. Eur J Neurosci. 1996 Mar;8(3):530-5. doi: 10.1111/j.1460-9568.1996.tb01237.x. Eur J Neurosci. 1996. PMID: 8963444 - Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease.
Martini R, Fischer S, López-Vales R, David S. Martini R, et al. Glia. 2008 Nov 1;56(14):1566-1577. doi: 10.1002/glia.20766. Glia. 2008. PMID: 18803324 Review. - Why is Wallerian degeneration in the CNS so slow?
Vargas ME, Barres BA. Vargas ME, et al. Annu Rev Neurosci. 2007;30:153-79. doi: 10.1146/annurev.neuro.30.051606.094354. Annu Rev Neurosci. 2007. PMID: 17506644 Review.
Cited by
- Cytokine expression in the epidural space: a model of noncompressive disc herniation-induced inflammation.
Cuéllar JM, Borges PM, Cuéllar VG, Yoo A, Scuderi GJ, Yeomans DC. Cuéllar JM, et al. Spine (Phila Pa 1976). 2013 Jan 1;38(1):17-23. doi: 10.1097/BRS.0b013e3182604baa. Spine (Phila Pa 1976). 2013. PMID: 22648034 Free PMC article. - Acute injury in the peripheral nervous system triggers an alternative macrophage response.
Ydens E, Cauwels A, Asselbergh B, Goethals S, Peeraer L, Lornet G, Almeida-Souza L, Van Ginderachter JA, Timmerman V, Janssens S. Ydens E, et al. J Neuroinflammation. 2012 Jul 20;9:176. doi: 10.1186/1742-2094-9-176. J Neuroinflammation. 2012. PMID: 22818207 Free PMC article. - Interleukin-1β influences functional regeneration following nerve injury in mice through nuclear factor-κB signaling pathway.
Wu R, Chen B, Jia X, Qiu Y, Liu M, Huang C, Feng J, Wu Q. Wu R, et al. Immunology. 2019 Mar;156(3):235-248. doi: 10.1111/imm.13022. Epub 2019 Jan 6. Immunology. 2019. PMID: 30418673 Free PMC article. - TNF-alpha and neuropathic pain--a review.
Leung L, Cahill CM. Leung L, et al. J Neuroinflammation. 2010 Apr 16;7:27. doi: 10.1186/1742-2094-7-27. J Neuroinflammation. 2010. PMID: 20398373 Free PMC article. Review. - Escalated regeneration in sciatic nerve crush injury by the combined therapy of human amniotic fluid mesenchymal stem cells and fermented soybean extracts, Natto.
Pan HC, Yang DY, Ho SP, Sheu ML, Chen CJ, Hwang SM, Chang MH, Cheng FC. Pan HC, et al. J Biomed Sci. 2009 Aug 23;16(1):75. doi: 10.1186/1423-0127-16-75. J Biomed Sci. 2009. PMID: 19698158 Free PMC article.
References
- Aggawal BB, Samanta A, Feldman M. TNFα. In: Oppenhheim JJ, Feldman M, editors. Cytokine reference. Academic; New York: 2001. pp. 413–434.
- Bandtlow CE, Schwab ME. NI-35/250/nogo-a: a neurite growth inhibitor restricting structural plasticity and regeneration of nerve fibers in the adult vertebrate CNS. Glia. 2000;29:175–181. - PubMed
- Be'eri H, Reichert F, Saada A, Rotshenker S. The cytokine network of wallerian degeneration: IL-10 and GM-CSF. Eur J Neurosci. 1998;10:2707–2713. - PubMed
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. - PubMed
- Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron. 1991;6:359–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases