A role for alpha-synuclein in the regulation of dopamine biosynthesis - PubMed (original) (raw)
A role for alpha-synuclein in the regulation of dopamine biosynthesis
Ruth G Perez et al. J Neurosci. 2002.
Erratum in
- J Neurosci 2002 Oct 15;22(20):9142
Abstract
The alpha-synuclein gene is implicated in the pathogenesis of Parkinson's disease. Although alpha-synuclein function is uncertain, the protein has homology to the chaperone molecule 14-3-3. In addition, alpha-synuclein can bind to 14-3-3, and both alpha-synuclein and 14-3-3 bind to many of the same proteins. Because 14-3-3 binds to and activates tyrosine hydroxylase, the rate-limiting enzyme in dopamine (DA) biosynthesis, we explored whether alpha-synuclein also bound to tyrosine hydroxylase and influenced its activity. Immunoprecipitation revealed an interaction between alpha-synuclein and tyrosine hydroxylase in brain homogenates and MN9D dopaminergic cells. Colocalization of alpha-synuclein with tyrosine hydroxylase was confirmed by immunoelectron microscopy. To explore the consequences of the interaction, we measured the effect of recombinant alpha-synuclein on tyrosine hydroxylase activity in a cell-free system and observed a dose-dependent inhibition of tyrosine hydroxylase by alpha-synuclein. To measure the impact of alpha-synuclein on tyrosine hydroxylase in dopaminergic cells, we stably transfected MN9D cells with wild-type or A53T mutant alpha-synuclein. Overexpression of wild-type or A53T mutant alpha-synuclein did not significantly alter tyrosine hydroxylase protein levels in our stably transfected cells. However, overexpressing cell lines had significantly reduced tyrosine hydroxylase activity and a corresponding reduction in dopamine synthesis. The reduction in cellular dopamine levels was not caused by increased dopamine catabolism or dopamine efflux. These data suggest that alpha-synuclein plays a role in the regulation of dopamine biosynthesis, acting to reduce the activity of tyrosine hydroxylase. If so, a loss of soluble alpha-synuclein, by reduced expression or aggregation, could increase dopamine synthesis with an accompanying increase in reactive dopamine metabolites.
Figures
Fig. 1.
Interaction of α-synuclein with TH in rat brain. Data from a representative experiment showing Western blots (WB) reacted with the α-synuclein antibody (a) or with the TH antibody (b). a, WB of α-synuclein from rat striatum shows α-synuclein in the initial homogenate (lane 1), the α-synuclein IP (Syn-IP) sample (lane 2), the TH-IP sample (lane 3), a control IP using preabsorbed TH antibody (Abs TH-IP, lane 4), and control IP using preabsorbed α-synuclein antibody (Abs Syn-IP,lane 5). In b the WB reacted with the TH antibody shows TH in the initial homogenate (lane 1), the α-synuclein IP sample (lane 2), the TH IP sample (lane 3), a control IP using preabsorbed TH antibody (lane 4), and control IP using preabsorbed α-synuclein antibody (lane 5). Nonspecific bands, two of which appear to be IgG bands, are evident in lanes with stronger chemiluminescence signal (indicated by asterisks in_a_ and b). Molecular weights in kilodaltons (kDa), determined from prestained standards, are indicated on the left.
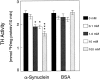
Fig. 2.
Inhibition of TH activity by α-synuclein_in vitro_. Unreacted3H-
l
-tyrosine was separated from3H2O using an acidic charcoal wash, and3H2O in the resulting supernatants was measured by scintillation counting for 10 min. Release of3H2O from 3H-
l
-tyrosine was diminished by recombinant α-synuclein in a dose-dependent manner using a cell-free in vitro assay. BSA did not significantly diminish TH activity at any dose. Data represent the mean ± SEM for five independent experiments using triplicate samples for each condition. *p < 0.05; ***p < 0.001.
Fig. 3.
Expression of α-synuclein and TH in parental and in stably transfected MN9D clonal cell lines. a, Parental MN9D (lane 1) and GFP-transfected MN9D cells (lane 2) express low endogenous α-synuclein levels compared with clonal cells overexpressing wild-type α-synuclein (WT Syn, lanes 3,4), or A53T mutant α-synuclein (lanes 5, 6) as determined by immunoblot of 20 μg protein per lane reacted with the Syn-1 anti-α-synuclein antibody. b, TH levels are equivalent in all MN9D cell lines whether expressing endogenous α-synuclein (parental_MN9D_, lane 1, or GFP-MN9D,lane 2) or overexpressing α-synuclein [wild-type (WT), lanes 3 and_4_, or A53T, lanes 5 and_6_] as determined by immunoblot of 20 μg protein per lane reacted with the MAB318 anti-TH antibody.
Fig. 4.
Interaction of α-synuclein with TH in MN9D cells. Western blots (WB) from a representative co-IP experiment reacted with the anti-α-synuclein antibody (a) or with the anti-TH antibody (b). Immunoprecipitation of α-synuclein from cell extracts (a) resulted in co-IP of TH (b) from parental MN9D (MN9D), GFP-transfected MN9D (GFP), wild-type (WT) α-synuclein, and A53T α-synuclein (A53T Syn) cells. Immunoelectron microscopy reveals colocalization of TH with α-synuclein that is apparent on mitochondria (c) and vesicles (d,e) in MN9D cells. In c and_d_, the larger (10 nm) gold particles label α-synuclein and the smaller (5 nm) particles label TH colocalized on the surface of a mitochondrion (c) and at the edge of a vesicular structure (d) in an MN9D cell stably transfected with A53T α-synuclein. In e the large (10 nm) gold particles label TH and the small (5 nm) particles label α-synuclein in the cytoplasm of an MN9D cell stably transfected with wild-type α-synuclein. Arrowheads in_c_–e point to colocalized small and large gold particles. Arrows in d point to the lipid bilayer of a vesicle decorated with large and small gold particles. m, Mitochondrion; v, vesicle. Scale bars, 100 nm.
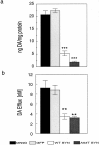
Fig. 5.
Effect of α-synuclein overexpression on cellular DA and DA efflux from MN9D cells. Supernatants obtained from cell lysates of parental MN9D, GFP, wild-type α-synuclein (WT SYN), and A53T mutant α-synuclein cells were assayed for DA using HPLC with electrochemical detection (a). Although A53T cells had even lower cellular DA levels than wild-type α-synuclein cells in a, the difference was not statistically significant using ANOVA with Tukey-Kramer post hoc analyses (p > 0.05). To measure DA efflux from the various MN9D lines, cells were washed in ACSF and incubated in fresh ACSF for 15 min at 37°C before collection (b). Data, normalized for total protein, represent the mean ± SEM of triplicate samples from two to six independent experiments.Black bar, MN9D; light gray bar, GFP;white bar, wild-type α-synuclein; dark gray bar, A53T α-synuclein. **p < 0.01; ***p < 0.001.
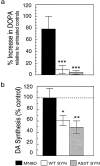
Fig. 6.
Effect of α-synuclein overexpression on DA synthesis in MN9D cell lines. Cells lysates were prepared from equivalent cultures of MN9D cells incubated in the presence of 200 μm NSD-1015 for 30 min. a, More DOPA accumulated after blocking AADC activity with NSD-1015 in parental MN9D cells than in wild-type (WT) or A53T mutant α-synuclein lines, indicating that TH activity is dramatically reduced in cells that overexpress α-synuclein. b, In situ DA synthesis was significantly greater for parental MN9D cells than for WT or A53T α-synuclein clonal lines incubated with
l
-[1-14C]tyrosine and compared for their ability to generate 14CO2 from the radioactive precursor. Data are from two to six independent experiments presented as the mean ± SEM for each condition. *p < 0.05; **p < 0.01; ***p < 0.001.
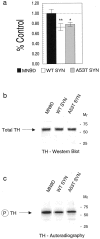
Fig. 7.
The impact of α-synuclein on TH phosphorylation in MN9D cells. Cells labeled with 32P were evaluated for TH phosphorylation by autoradiography of cell lysates from control MN9D and stably transfected α-synuclein lines separated using SDS-PAGE and analyzed by phosphorimaging (a). After TH immunoprecipitation from cell lysates, total TH levels (at_arrow_) from a representative experiment appear similar for MN9D, WT, and A53T MN9D lines as determined by Western blot reacted with the AB151 anti-TH antibody and visualized by chemiluminescence (b). Phospho-TH levels (at_arrow_ in c) are significantly reduced in cells overexpressing wild-type α-synuclein or A53T mutant α-synuclein compared with control MN9D cells as seen by autoradiography. In a, black bar_represents MN9D, w_hite bar represents wild-type α-synuclein, and gray bar represents A53T α-synuclein. Molecular weights, determined from prestained standards, are indicated on the right in b and_c_. *p < 0.05; **p < 0.01.
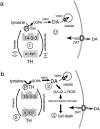
Fig. 8.
The potential roles of α-synuclein in dopamine synthesis (a) and neurodegeneration (b). DA is synthesized in a multistep process in which the amino acid tyrosine is converted to DOPA by the activity of phosphorylated TH and aromatic amino acid decarboxylase (AADC). 14-3-3 is known to bind to TH that is phosphorylated and α-synuclein appears to bind to dephospho-TH. 14-3-3 and α-synuclein may differentially regulate TH activity (at_1_ in a) to maintain optimal DA levels in concert with the activities of the vesicular monoamine transporter (VMAT2, indicated by a small box on vesicle), which normally packages intracellular DA into vesicles, and the dopamine transporter (DAT, indicated by large white box outlined in black on the plasma membrane), which can function bidirectionally. α-Synuclein may directly bind to TH and inhibit TH activity and/or phosphorylation, or α-synuclein may act indirectly by activating a phosphatase (indicated by + in a) or inhibiting a kinase (indicted by − in a) to affect TH phosphorylation. In b, a reduction in free soluble α-synuclein (indicated by gray dashed X over α-syn, at 2) may occur by downregulation of α-synuclein mRNA or by stimuli that induce fibrilization (e.g., environmental toxins, mutation, nitration, or ubiquitination). Disinhibition of TH may lead to elevated cytosolic DA in neurons with subsequent generation of DA-quinone and DA-related ROS (at 3 in b), which can damage proteins, lipids, and DNA and contribute to neurotoxicity.
Similar articles
- Phosphorylation of α-synuclein upregulates tyrosine hydroxylase activity in MN9D cells.
Wu B, Liu Q, Duan C, Li Y, Yu S, Chan P, Uéda K, Yang H. Wu B, et al. Acta Histochem. 2011 Jan;113(1):32-5. doi: 10.1016/j.acthis.2009.07.007. Epub 2009 Aug 14. Acta Histochem. 2011. PMID: 19683335 - Could a loss of alpha-synuclein function put dopaminergic neurons at risk?
Perez RG, Hastings TG. Perez RG, et al. J Neurochem. 2004 Jun;89(6):1318-24. doi: 10.1111/j.1471-4159.2004.02423.x. J Neurochem. 2004. PMID: 15189334 Review. - Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells.
Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Peng X, et al. J Cell Sci. 2005 Aug 1;118(Pt 15):3523-30. doi: 10.1242/jcs.02481. Epub 2005 Jul 19. J Cell Sci. 2005. PMID: 16030137 - Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells.
Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG. Tehranian R, et al. J Neurochem. 2006 Nov;99(4):1188-96. doi: 10.1111/j.1471-4159.2006.04146.x. Epub 2006 Sep 18. J Neurochem. 2006. PMID: 16981894 - Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse?
Sidhu A, Wersinger C, Vernier P. Sidhu A, et al. FASEB J. 2004 Apr;18(6):637-47. doi: 10.1096/fj.03-1112rev. FASEB J. 2004. PMID: 15054086 Review.
Cited by
- 14-3-3 proteins-a moonlight protein complex with therapeutic potential in neurological disorder: in-depth review with Alzheimer's disease.
Abdi G, Jain M, Patil N, Upadhyay B, Vyas N, Dwivedi M, Kaushal RS. Abdi G, et al. Front Mol Biosci. 2024 Feb 5;11:1286536. doi: 10.3389/fmolb.2024.1286536. eCollection 2024. Front Mol Biosci. 2024. PMID: 38375509 Free PMC article. Review. - Dopamine Signaling in Substantia Nigra and Its Impact on Locomotor Function-Not a New Concept, but Neglected Reality.
Salvatore MF. Salvatore MF. Int J Mol Sci. 2024 Jan 17;25(2):1131. doi: 10.3390/ijms25021131. Int J Mol Sci. 2024. PMID: 38256204 Free PMC article. Review. - Genetic influences on craving for alcohol.
Agrawal A, Wetherill L, Bucholz KK, Kramer J, Kuperman S, Lynskey MT, Nurnberger JI Jr, Schuckit M, Tischfield JA, Edenberg HJ, Foroud T, Bierut LJ. Agrawal A, et al. Addict Behav. 2013 Feb;38(2):1501-1508. doi: 10.1016/j.addbeh.2012.03.021. Epub 2012 Mar 19. Addict Behav. 2013. PMID: 22481050 Free PMC article. - TFEB and TFE3: Linking Lysosomes to Cellular Adaptation to Stress.
Raben N, Puertollano R. Raben N, et al. Annu Rev Cell Dev Biol. 2016 Oct 6;32:255-278. doi: 10.1146/annurev-cellbio-111315-125407. Epub 2016 Jun 1. Annu Rev Cell Dev Biol. 2016. PMID: 27298091 Free PMC article. Review. - Generation of G51D and 3D mice reveals decreased α-synuclein tetramer-monomer ratios promote Parkinson's disease phenotypes.
Nuber S, Zhang X, McCaffery TD, Moors TE, Adom MA, Hahn WN, Martin D, Ericsson M, Tripathi A, Dettmer U, Svenningsson P, Selkoe DJ. Nuber S, et al. NPJ Parkinsons Dis. 2024 Feb 29;10(1):47. doi: 10.1038/s41531-024-00662-w. NPJ Parkinsons Dis. 2024. PMID: 38424059 Free PMC article.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington: clinical, morphological, and neurochemical correlations. J Neurol Sci. 1973;20:415–455. - PubMed
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. - PubMed
- Bevilaqua LR, Graham ME, Dunkley PR, von Nagy-Felsobuki EI, Dickson PW. Phosphorylation of ser19 alters the conformation of tyrosine hydroxylase to increase the rate of phosphorylation of ser40. J Biol Chem. 2001;276:40411–40416. - PubMed
- Broadie K, Rushton E, Skoulakis EM, Davis RL. Leonardo, a Drosophila 14-3-3 protein involved in learning, regulates presynaptic function. Neuron. 1997;19:391–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases