Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors - PubMed (original) (raw)
Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors
Francoise Rouge-Pont et al. J Neurosci. 2002.
Abstract
An increase of extracellular dopamine (DA) concentration is a major neurobiological substrate of the addictive properties of drugs of abuse. In this article we investigated the contribution of the DA D2 receptor (D2R) in the control of this response. Extracellular DA levels were measured in the striatum of mice lacking D2R expression (D2R-/-) by in vivo microdialysis after administration of the psychostimulant cocaine and the opioid morphine. Interestingly, the increase in extracellular DA induced by both drugs was strikingly higher in D2R-/- than in wild-type littermates. This indicates that D2Rs play a key role in the modulation of DA release in response to drugs of abuse. Furthermore, this observation prompted us to investigate the dopaminergic autoreceptor function in the absence of D2 receptor in D2R-/- mice. Results obtained using complementary microdialysis and voltammetry analyses show that the autoreceptor function regulating DA release is totally abolished in the absence of D2R, despite unchanged DA uptake and basal DA efflux. Finally, we propose that the short isoform D2S receptor of the D2 receptors is the one controlling change in DA release induced by drugs of abuse. Indeed, the neurochemical effects of cocaine and morphine are unchanged in animals with a selective deletion of the long isoform D2L receptor. Thus, deregulated expression of D2R isoforms might be involved in the vulnerability of an individual to drug abuse.
Figures
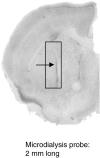
Fig. 1.
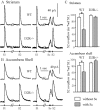
Position of the microdialysis probe in the dorsal striatum. Coronal section (30 μm) of a mouse brain showing the implantation site of a dialysis probe, in the dorsal striatum (arrow), and the area within which all probes were contained (rectangle).
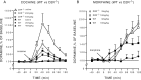
Fig. 2.
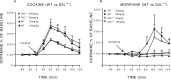
Cocaine- and morphine-induced changes in extracellular concentration of dopamine in WT and D2R−/− mice.A, The administration of cocaine (20 and 40 mg/kg, i.p) induced a higher increase in dopamine in D2R−/− than in WT animals.B, The administration of morphine (9 and 18 mg/kg, s.c.) induced a higher increase in dopamine in D2R−/− than in WT mice. Dialysates were collected in the striatum of freely moving animals every 20 min, and data are expressed as mean ± SEM of changes from baseline.
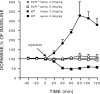
Fig. 3.
Quinpirole- and haloperidol-induced changes in extracellular concentration of dopamine in WT and D2R−/− mice. The D2-specific agonist (quinpirole) and antagonist (haloperidol) were administered in WT and D2R−/− mice. Quinpirole and haloperidol, respectively, decrease and increased DA release in WT animals. In contrast, the two drugs had no effects in D2R −/− mice. Dialysates were collected in the striatum of freely moving animals every 20 min, and data are expressed as mean ± SEM of changes from baseline.
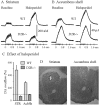
Fig. 4.
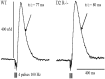
Effect of haloperidol on the DA overflow evoked by prolonged stimulations in striatum and AcbSh of WT and D2R−/− mice. Typical recordings of the effect of haloperidol on DA overflow in one WT mouse and one D2R −/− mouse before (Baseline) and 20 min after haloperidol (0.5 mg/kg, s.c.). DA overflow was evoked by MFB stimulation consisting of 10 pulses at 15 Hz. The DA overflow recorded in striatum (A) was expressed in terms of changes in DA concentration by in vitro calibration of the electrode. In AcbSh (B), because the carbon fiber electrode was not calibrated after each experiment, the DA overflow was expressed in terms of changes in oxidation current. The maximal amplitude of the DA overflow observed 20 min after haloperidol was expressed as a percentage of that observed before injection (C). Data are given as mean ± SEM (5 animals in each group in the striatum, 6 WT and 4 D2R−/− mice in AcbSh). Anatomical location of the electrode tip in the dorsal striatum and accumbens shell (D).
Fig. 5.
Autoregulation of the evoked DA release in striatum and AcbSh of WT and D2R−/− mice. The DA overflow was evoked in striatum (A) and AcbSh (B) by MFB electrical stimulation. Test stimulations S1 and S2 consisted of three pulses at 100 Hz and were applied 4 sec apart. The conditioning stimulation (Sc) consisted of four pulses at 15 Hz, and the delay between the end of Sc and S2 was 300 msec. The amplitude of the DA overflow evoked by S2 was expressed as a percentage of the overflow evoked by S1 and reflected the inhibition of DA release induced by Sc. The data are given as mean ± SEM (8 WT and 6 D2R−/− in striatum, 6 WT and 5 D2R−/− in the shell). In the absence of conditioning stimulation (noSc), S1 and S2 were similar in WT and D2R−/− mice. In WT mice the DA overflow evoked by Sc inhibited DA release evoked by S2 (*p< 0.002). In contrast, in D2R −/− mice Sc did not modify further DA release.
Fig. 6.
DA overflow evoked in striatum of WT and D2R −/−mice. DA overflow was evoked in the dorsal striatum by MFB electrical stimulation consisting of four pulses at 100 Hz. In each animal the evoked DA overflow was measured with the same electrode at three different depths from the cortical surface (2.75, 3, and 3.25 mm). DA overflow was expressed in terms of change in dopamine concentration by calibrating the electrode in vitro at the end of each experiment. For each individual overflow two parameters were measured: the half-life (i.e., the time for 50% decrease from the maximum) and the maximal amplitude. The three pairs of values obtained from the same animal were averaged. Data collected from several experiments are given in Table 1.
Fig. 7.
Cocaine and morphine-induced changes in extracellular concentration of dopamine in WT and D2L−/− mice.A, The administration of cocaine (20 and 40 mg/kg, i.p) and morphine (B) (9 and 18 mg/kg, s.c.) induced a similar increase in dopamine in D2L−/− and in WT animals. Dialysates were collected in the striatum of freely moving animals every 20 min, and data are expressed as mean ± SEM of changes from baseline.
Similar articles
- Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum.
Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dickinson SD, et al. J Neurochem. 1999 Jan;72(1):148-56. doi: 10.1046/j.1471-4159.1999.0720148.x. J Neurochem. 1999. PMID: 9886065 - Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo.
Benoit-Marand M, Borrelli E, Gonon F. Benoit-Marand M, et al. J Neurosci. 2001 Dec 1;21(23):9134-41. doi: 10.1523/JNEUROSCI.21-23-09134.2001. J Neurosci. 2001. PMID: 11717346 Free PMC article. - Lack of autoreceptor-mediated inhibitory control of dopamine release in striatal synaptosomes of D2 receptor-deficient mice.
L'hirondel M, Chéramy A, Godeheu G, Artaud F, Saiardi A, Borrelli E, Glowinski J. L'hirondel M, et al. Brain Res. 1998 May 11;792(2):253-62. doi: 10.1016/s0006-8993(98)00146-2. Brain Res. 1998. PMID: 9593923 - Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice.
Koeltzow TE, Xu M, Cooper DC, Hu XT, Tonegawa S, Wolf ME, White FJ. Koeltzow TE, et al. J Neurosci. 1998 Mar 15;18(6):2231-8. doi: 10.1523/JNEUROSCI.18-06-02231.1998. J Neurosci. 1998. PMID: 9482807 Free PMC article. Review. - Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum.
Loonam TM, Noailles PA, Yu J, Zhu JP, Angulo JA. Loonam TM, et al. Life Sci. 2003 Jun 27;73(6):727-39. doi: 10.1016/s0024-3205(03)00393-x. Life Sci. 2003. PMID: 12801594 Review.
Cited by
- Longitudinal changes of dopamine transporters in heroin users during abstinence.
Xu S, Liu Y, Li Y, Deng Y, Huang Y, Yuan J, Lv R, Wang Y, Zhang G, Guo Z, Han M, Liu X, Fu D. Xu S, et al. Psychopharmacology (Berl). 2015 Sep;232(18):3391-401. doi: 10.1007/s00213-015-3992-0. Epub 2015 Jun 23. Psychopharmacology (Berl). 2015. PMID: 26096461 Clinical Trial. - Parkinsonism Driven by Antipsychotics Originates from Dopaminergic Control of Striatal Cholinergic Interneurons.
Kharkwal G, Brami-Cherrier K, Lizardi-Ortiz JE, Nelson AB, Ramos M, Del Barrio D, Sulzer D, Kreitzer AC, Borrelli E. Kharkwal G, et al. Neuron. 2016 Jul 6;91(1):67-78. doi: 10.1016/j.neuron.2016.06.014. Neuron. 2016. PMID: 27387649 Free PMC article. - Effect of sub-chronic hydrocortisone on responses to amphetamine in normal male volunteers.
Hearn AJ, Gallagher P, Owen BM, Smith MS, Watson S, Young AH. Hearn AJ, et al. Psychopharmacology (Berl). 2004 Feb;171(4):458-64. doi: 10.1007/s00213-003-1609-5. Epub 2003 Sep 23. Psychopharmacology (Berl). 2004. PMID: 14504680 Clinical Trial. - Dopamine involved in the nociceptive modulation in the parafascicular nucleus of morphine-dependent rat.
Gao HR, Shi TF, Yang CX, Zhang GW, Zhang D, Jiao RS, Zhang Y, Xu MY, Zhu H. Gao HR, et al. Neurochem Res. 2012 Feb;37(2):428-35. doi: 10.1007/s11064-011-0629-5. Epub 2011 Oct 14. Neurochem Res. 2012. PMID: 21996785 - Getting specialized: presynaptic and postsynaptic dopamine D2 receptors.
De Mei C, Ramos M, Iitaka C, Borrelli E. De Mei C, et al. Curr Opin Pharmacol. 2009 Feb;9(1):53-8. doi: 10.1016/j.coph.2008.12.002. Epub 2009 Jan 8. Curr Opin Pharmacol. 2009. PMID: 19138563 Free PMC article. Review.
References
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. - PubMed
- Benoit-Marand M, Jaber M, Gonon F. Release and elimination of dopamine in vivo in mice lacking the dopamine transporter: functional consequences. Eur J Neurosci. 2000;12:2985–2992. - PubMed
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. - PubMed
- Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176:259–263. - PubMed
- Devoto P, Collu M, Muntoni AL, Pistis M, Serra G, Gessa GL, Diana M. Biochemical and electrophysiological effects of 7-OH-DPAT on the mesolimbic dopaminergic system. Synapse. 1995;20:153–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases