Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes - PubMed (original) (raw)
Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes
Martin Sachse et al. Mol Biol Cell. 2002 Apr.
Abstract
In many cells endosomal vacuoles show clathrin coats of which the function is unknown. Herein, we show that this coat is predominantly present on early endosomes and has a characteristic bilayered appearance in the electron microscope. By immunoelectron microscopy we show that the coat contains clathrin heavy as well as light chain, but lacks the adaptor complexes AP1, AP2, and AP3, by which it differs from clathrin coats on endocytic vesicles and recycling endosomes. The coat is insensitive to short incubations with brefeldin A, but disappears in the presence of the phosphatidylinositol 3-kinase inhibitor wortmannin. No association of endosomal coated areas with tracks of tubulin or actin was found. By quantitative immunoelectron microscopy, we found that the lysosomal-targeted receptors for growth hormone (GHR) and epidermal growth factor are concentrated in the coated membrane areas, whereas the recycling transferrin receptor is not. In addition, we found that the proteasomal inhibitor MG 132 induces a redistribution of a truncated GHR (GHR-369) toward recycling vesicles, which coincided with a redistribution of endosomal vacuole-associated GHR-369 to the noncoated areas of the limiting membrane. Together, these data suggest a role for the bilayered clathrin coat on vacuolar endosomes in targeting of proteins to lysosomes.
Figures
Figure 1
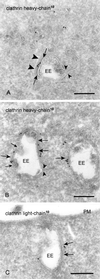
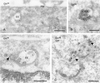
Bilayered coats on endosomal vacuoles contain clathrin heavy and light chain. (A) Chinese hamster ovary cell. Clathrin heavy chain (10-nm gold) is found on the flat, coated region of an EE. The clathrin coat consists of a narrow electron-dense layer opposed to the endosomal limiting membrane (arrows) and a fuzzier layer facing the cytoplasm (arrowheads). Note that the recycling tubule emerges opposite to the coated region (small arrowheads). (B) Also in wtGHR cells, clathrin heavy chain (10-nm gold) is found in the coated areas (arrows). Note that no label is found on the inward budding vesicles (small arrows). As in A the emerging tubule has no continuity with the coated region (small arrowheads). (C) Example of an early endosome in wtGHR cells, labeled for clathrin light chain (10-nm gold) in the coated areas (arrows). PM, plasma membrane. Bars, 200 nm.
Figure 2
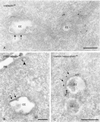
Clathrin adaptor complexes AP1 and AP2 are absent from the bilayered coats. (A) HeLa cells labeled for γ-adaptin (10-nm gold). Label is present on vesicles and tubules in vicinity to EEs but absent from the bilayered coat (arrows). (B) WtGHR cells labeled for β1/β2-adaptin (10-nm gold). Label is found at a clathrin-coated pit (arrowhead) but not in the bilayered coat (arrows). (C) WtGHR cells incubated for 10 min with 10 mM brefeldin A and labeled for clathrin heavy chain (10-nm gold). At this time point brefeldin A does not interfere with the bilayered coat (arrows). LE, late endosome; PM, plasma membrane. Bars, 200 nm.
Figure 3
Wortmannin causes the disappearance of the bilayered coats from endosomes. WtGHR cells incubated for 45 min with 100 nM wortmannin. (A) Wortmannin induces swollen endosomal vacuoles (V) that rarely bear a visible bilayered coat and are not labeled for clathrin (10-nm gold). In contrast, association of clathrin with cytoplasmic vesicles and tubules is unperturbed (arrowheads). (B) Early endosomal marker TfR (10-nm gold) localizes to the limiting membrane of swollen endosomal vacuoles. Bars, 200 nm.
Figure 4
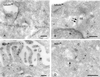
Bilayered coats are not associated with microtubules or actin (A) HeLa cells. Lysosomes (L) are associated with a linear track of tubulin (10-nm gold). (B) In contrast, tubulin is not associated with the coated areas (arrows) on EEs. (C) Actin (10-nm gold) is readily found near the plasma membrane (PM) and in microvillar protrusions. (D) When actin is found near the limiting membrane of EEs, it is not associated with coated areas (arrow). Bars, 200 nm.
Figure 5
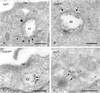
EGFR, Hrs, and GHR are concentrated in the bilayered coats. (A and B) WtGHR cell incubated for 30 min with biotinylated GH. (A) TfR (10-nm gold) label occurs predominantly in REs, some of which are in vicinity of early endosomes (small arrowheads). At the limiting membrane of the EEs TfR is localized in coated (arrows) and noncoated areas (arrowhead). (B) GHR (10-nm gold) is present at the limiting membrane as well as on internal vesicles of an EE. At the limiting membrane, GHR is found in coated areas (arrows). (C and D) HeLa cells incubated for 10 min with EGF. (C) EGFR (10-nm gold) is clearly present in coated areas (arrows) of an EE. (D) Also Hrs (10-nm gold) is localized in coated areas (arrows) of EEs. PM, plasma membrane. Bars, 200 nm.
Figure 6
MG 132 induces recycling of endocytosed GHR-369. (A and B) GHR-369 cells incubated for 1 h with biotinylated GH. (A) GH (10-nm gold) is found on the plasma membrane (PM), as well as in EEs. (B) Example of GH (10-nm gold) staining on the internal vesicles of an LE. (C) GHR-369–expressing cells incubated for 1 h in the presence of MG 132 and subsequently 1 h with MG 132 plus biotinylated GH. GH (10-nm gold) is found on both the limiting membrane and internal vesicles of an EE. Note that label is present in a tubule that evolves from the vacuole (arrowhead). (D) Cells were treated as in C but GH was used instead of biotinylated ligand and in the last 30 min also biotinylated Tf was added. Double labeling for GH (10-nm gold) and biotin (15-nm gold) showed colocalization on electron-dense REs (arrowheads). N, nucleus. Bars, 200 nm.
Figure 7
Syntaxin 7 is concentrated in the bilayered coat (A) WtGHR cells. Syntaxin 7 (10-nm gold) is largely restricted to the limiting membrane of EEs, where it accumulates in the bilayered coated areas (arrows). (B) In HeLa cells, syntaxin 7 (10-nm gold) shows a similar staining. Bars, 200 nm.
Figure 8
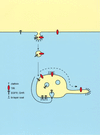
Converging lines of evidence suggest a model, in which an interaction between coat components and down-regulated receptors results in retention in the bilayered coats. This retention concentrate lysosomal routed proteins and segregates them from recycling proteins. Recycling proteins that are not retained in the bilayered coats pass through the endosomal vacuole and follow the massive bulk flow into recycling endosomes (dashed bold line). In contrast, retention in the coats (continuous bold line) of proteins routed to lysosomes would precede incorporation in internal endosomal vesicles and subsequent transport to lysosomes.
Similar articles
- Clathrin hub expression affects early endosome distribution with minimal impact on receptor sorting and recycling.
Bennett EM, Lin SX, Towler MC, Maxfield FR, Brodsky FM. Bennett EM, et al. Mol Biol Cell. 2001 Sep;12(9):2790-9. doi: 10.1091/mbc.12.9.2790. Mol Biol Cell. 2001. PMID: 11553717 Free PMC article. - NECAP2 controls clathrin coat recruitment to early endosomes for fast endocytic recycling.
Chamberland JP, Antonow LT, Dias Santos M, Ritter B. Chamberland JP, et al. J Cell Sci. 2016 Jul 1;129(13):2625-37. doi: 10.1242/jcs.173708. Epub 2016 May 20. J Cell Sci. 2016. PMID: 27206861 - ATPase-deficient hVPS4 impairs formation of internal endosomal vesicles and stabilizes bilayered clathrin coats on endosomal vacuoles.
Sachse M, Strous GJ, Klumperman J. Sachse M, et al. J Cell Sci. 2004 Apr 1;117(Pt 9):1699-708. doi: 10.1242/jcs.00998. Epub 2004 Mar 9. J Cell Sci. 2004. PMID: 15075231 - Connecting the dots: combined control of endocytic recycling and degradation.
MacDonald E, Savage B, Zech T. MacDonald E, et al. Biochem Soc Trans. 2020 Dec 18;48(6):2377-2386. doi: 10.1042/BST20180255. Biochem Soc Trans. 2020. PMID: 33300959 Free PMC article. Review. - Revising Endosomal Trafficking under Insulin Receptor Activation.
Iraburu MJ, Garner T, Montiel-Duarte C. Iraburu MJ, et al. Int J Mol Sci. 2021 Jun 29;22(13):6978. doi: 10.3390/ijms22136978. Int J Mol Sci. 2021. PMID: 34209489 Free PMC article. Review.
Cited by
- Melanosomes--dark organelles enlighten endosomal membrane transport.
Raposo G, Marks MS. Raposo G, et al. Nat Rev Mol Cell Biol. 2007 Oct;8(10):786-97. doi: 10.1038/nrm2258. Nat Rev Mol Cell Biol. 2007. PMID: 17878918 Free PMC article. Review. - Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation.
Razi M, Futter CE. Razi M, et al. Mol Biol Cell. 2006 Aug;17(8):3469-83. doi: 10.1091/mbc.e05-11-1054. Epub 2006 May 17. Mol Biol Cell. 2006. PMID: 16707569 Free PMC article. - Exploring the ESCRTing machinery in eukaryotes.
Winter V, Hauser MT. Winter V, et al. Trends Plant Sci. 2006 Mar;11(3):115-23. doi: 10.1016/j.tplants.2006.01.008. Epub 2006 Feb 20. Trends Plant Sci. 2006. PMID: 16488176 Free PMC article. Review. - Intracellular mediators of transforming growth factor beta superfamily signaling localize to endosomes in chicken embryo and mouse lenses in vivo.
Rajagopal R, Ishii S, Beebe DC. Rajagopal R, et al. BMC Cell Biol. 2007 Jun 25;8:25. doi: 10.1186/1471-2121-8-25. BMC Cell Biol. 2007. PMID: 17592637 Free PMC article. - A protein interaction network for Ecm29 links the 26 S proteasome to molecular motors and endosomal components.
Gorbea C, Pratt G, Ustrell V, Bell R, Sahasrabudhe S, Hughes RE, Rechsteiner M. Gorbea C, et al. J Biol Chem. 2010 Oct 8;285(41):31616-33. doi: 10.1074/jbc.M110.154120. Epub 2010 Aug 3. J Biol Chem. 2010. PMID: 20682791 Free PMC article.
References
- Bentham J, Aplin R, Norman MR. Histochemical detection of binding sites for human growth hormone using biotinylated ligand. J Histochem Cytochem. 1994;42:103–107. - PubMed
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999;1:249–252. - PubMed
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. - PubMed
- de Melker AA, van Der Horst G, Calafat J, Jansen H, Borst J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane, and remains receptor associated throughout the endocytic route. J Cell Sci. 2001;114:2167–2178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials