Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes - PubMed (original) (raw)
Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes
Joanne Layland et al. J Physiol. 2002.
Abstract
Nitric oxide (NO) can directly modulate cardiac contractility by accelerating relaxation and reducing diastolic tone. The intracellular mechanisms underlying these contractile effects are poorly understood. Here we investigate the role of cyclic GMP-dependent protein kinase (PKG) in the contractile response to exogenous NO in rat ventricular myocytes. Isolated ventricular myocytes were stimulated electrically and contractility was assessed by measuring cell shortening. Some cells were loaded with the fluorescent Ca(2+) probe indo-1 AM for simultaneous assessment of the intracellular Ca(2+) transient. The NO donor diethylamine NONOate (DEA/NO, 10 microM) significantly increased resting cell length, reduced twitch amplitude and accelerated time to 50 % relaxation (to 100.8 +/- 0.2, 83.7 +/- 3.0 and 88.9 +/- 3.7 % of control values, respectively). The contractile effects of DEA/NO occurred without significant changes in the amplitude or kinetics of the intracellular Ca(2+) transient, suggesting that the myofilament response to Ca(2+) was reduced. These effects were abolished by inhibition of either guanylyl cyclase (with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; ODQ, 10 microM) or PKG (with Rp-8-Br-cGMPs, 10 microM) suggesting that, at the concentration investigated, the effects of DEA/NO were mediated exclusively by PKG, following activation of guanylyl cyclase and elevation of cGMP. Direct activation of PKG with 8-pCPT-cGMP (10 microM) mimicked the effects of DEA/NO (resting cell length and time to 50 % relaxation were 100.6 +/- 0.1 and 90.5 +/- 1.5 % of control values, respectively).The reduced myofilament Ca(2+) responsiveness was not attributable to an intracellular acidosis since the small reduction in pH(i) induced by DEA/NO was found to be uncoupled from its contractile effects. However, hearts treated with DEA/NO (10 microM) showed a significant increase (1.4-fold; P < 0.01) in troponin I phosphorylation compared to control, untreated hearts. These results suggest that the reduction in myofilament Ca(2+) responsiveness produced by DEA/NO results from phosphorylation of troponin I by PKG.
Figures
Figure 1. Dose-dependent relaxant and negative inotropic effects of DEA/NO in isolated cardiac myocytes
A, twitch contractions from a typical cell. Numbers next to each trace indicate the concentration of DEA/NO applied (μ
m
). _B_-E, mean data (±
s.e.m.
) for changes in resting cell length, twitch amplitude, time to peak shortening (_T_pk), and time from peak shortening to 50 % relaxation (RT50). Control data (0 DEA/NO) is from a total of 18 cells investigated. Note that the effects of lower concentrations (10−9 and 10−8
m
) and higher concentrations (10−7 to 10−4
m
) of DEA/NO were investigated in separate group of cells (_n_≥ 9 cells for each concentration) but there were no significant differences in the control data between these groups (unpaired t test, P > 0.05). *Statistical significance compared to corresponding control data (paired t tests, P < 0.05).
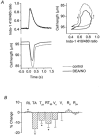
Figure 2. Reduction in myofilament Ca2+ responsiveness induced by DEA/NO (10 μm)
A, left panel, representative example of effect on myocyte contraction and simultaneous intracellular Ca2+ transients (indo-1 410/480 nm ratio); right panel, phase-plane loops of cell length against intracellular Ca2+ for the twitches shown in left panel. Thin lines denote control conditions, and thick lines denote data recorded after 10 min exposure to DEA/NO. B, averaged percentage changes relative to the control value (mean ±
s.e.m.
) in contractile parameters and indo-1 410/480 nm ratio following application of DEA/NO. *P < 0.05. Numbers in parentheses indicate the number of cells averaged. Actual values (mean ±
s.e.m.
) are given in Table 1. RL, resting cell length (n = 15); TA, twitch amplitude (n = 15); _T_pk, time to peak shortening (n = 15); RT50, time from minimum length to half relaxation (n = 15); _V_s, maximum shortening velocity (n = 15); _V_l, maximum re-lengthening velocity (n = 15); _R_d, diastolic indo-1 410/480 nm ratio (n = 7); _R_pk, peak indo-1 410/480 nm ratio (n = 7).
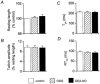
Figure 3. Effect of the guanylyl cyclase inhibitor ODQ on responses to DEA/NO (10 μm)
_A_-D, mean data (±
s.e.m.
) for changes in resting cell length, twitch amplitude, time to peak shortening (_T_pk), and time from peak shortening to 50 % relaxation (RT50). Open columns, values prior to addition of any drugs; hatched columns, ODQ alone; filled columns, DEA/NO in the presence of ODQ. n = 20.
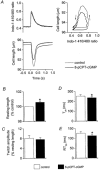
Figure 4. Effect of the PKG inhibitor Rp-8-Br-cGMPs (10 μm) on responses to DEA/NO (10 μm)
A, left panel, twitch contractions and simultaneous intracellular Ca2+ transients from a typical cell in control conditions (thin lines), after addition of Rp-8-Br-cGMPs (dotted lines), and following application of DEA/NO in the continued presence of Rp-8-Br-cGMPs (thick lines); right panel, phase-plane loops of cell length against intracellular Ca2+ for the twitches shown in left panel. _B_-E, mean data (±
s.e.m.
) for changes in resting cell length, twitch amplitude, time to peak shortening (_T_pk), and time from peak shortening to 50 % relaxation (RT50). Open columns, values prior to addition of any drugs; hatched columns, Rp-8-Br-cGMPs alone; filled columns, DEA/NO in the presence of Rp-8-Br-cGMPs. n = 10.
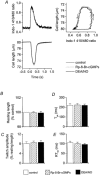
Figure 5. Effect of 8-pCPT-cGMP (10 μm) in isolated cardiac myocytes
A, left panel, representative example of effect on myocyte contraction and intracellular Ca2+ transients; right panel, phase-plane loops of cell length against intracellular Ca2+ for the twitches shown in left panel. _B_-E, mean data (±
s.e.m.
) for changes in resting cell length, twitch amplitude, time to peak shortening (_T_pk), and time from peak shortening to 50 % relaxation (RT50). Open columns, values prior to addition of any drugs; filled columns, after 8-pCPT-cGMP. n = 14; *P < 0.05.
Figure 6. Effect of DEA/NO (10 μm) on pHi in C-SNARF-1-loaded ventricular myocytes
A, effect of time alone (open squares, n = 35 cells) and DEA/NO added at 10 min (filled circles, n = 36 cells). Arrow indicates DEA/NO application. *P < 0.05 compared to the effects of time alone (unpaired t test). B, mean (±
s.e.m.
) data for changes in pHi with DEA/NO alone (filled columns), DEA/NO in the presence of Rp-8-Br-cGMPs (hatched columns), or DEA/NO in the presence of HOE-642 (open columns). Numbers in brackets indicate the number of cells averaged. *P < 0.05.
Figure 7. Effect of the Na+-H+ exchange inhibitor HOE-642 (3 μm) on responses to DEA/NO (10 μm)
A, twitch contractions from a typical cell in control conditions (thin lines), after addition of HOE-642 (dotted lines), and following application of DEA/NO in the continued presence of HOE-642 (thick lines). _B_-E, mean data (±
s.e.m.
) for changes in resting cell length, twitch amplitude, time to peak shortening (_T_pk), and time from peak shortening to 50 % relaxation (RT50). Open columns, values prior to addition of any drugs; hatched columns, HOE-642 alone; filled columns, DEA/NO in the presence of HOE-642. *P < 0.05 compared to control or HOE-642 alone.
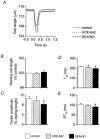
Figure 8. Immunoblots for total troponin I (A) and phosphorylated troponin I (B) in LV myocardium
Left panel, hearts treated with either DEA/NO (10 μ
m
) or vehicle alone (control). Right panel, hearts treated with either isoproterenol (Iso, 3 μ
m
) or vehicle alone (control).
Similar articles
- Nitric oxide effects depend on different mechanisms in different regions of the rat heart.
Derici K, Samsar U, Demirel-Yilmaz E. Derici K, et al. Heart Vessels. 2012 Jan;27(1):89-97. doi: 10.1007/s00380-011-0116-6. Epub 2011 Feb 11. Heart Vessels. 2012. PMID: 21311889 - Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes.
Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Vila-Petroff MG, et al. Circ Res. 1999 May 14;84(9):1020-31. doi: 10.1161/01.res.84.9.1020. Circ Res. 1999. PMID: 10325239 Free PMC article. - cGMP-dependent protein kinase in regulation of basal tone and in nitroglycerin- and nitric-oxide-induced relaxation in porcine coronary artery.
Qin X, Zheng X, Qi H, Dou D, Raj JU, Gao Y. Qin X, et al. Pflugers Arch. 2007 Sep;454(6):913-23. doi: 10.1007/s00424-007-0249-8. Epub 2007 Mar 22. Pflugers Arch. 2007. PMID: 17377806 - The influence of endothelium-derived nitric oxide on myocardial contractile function.
Shah AM, Prendergast BD, Grocott-Mason R, Lewis MJ, Paulus WJ. Shah AM, et al. Int J Cardiol. 1995 Jul;50(3):225-31. doi: 10.1016/0167-5273(95)02381-6. Int J Cardiol. 1995. PMID: 8537145 Review. - Regulation of basal myocardial function by NO.
Kojda G, Kottenberg K. Kojda G, et al. Cardiovasc Res. 1999 Mar;41(3):514-23. doi: 10.1016/s0008-6363(98)00314-9. Cardiovasc Res. 1999. PMID: 10435023 Review.
Cited by
- Genes of the cGMP-PKG-Ca2+ signaling pathway are alternatively spliced in cardiomyopathy: Role of RBFOX2.
Wan X, Belanger K, Widen SG, Kuyumcu-Martinez MN, Garg NJ. Wan X, et al. Biochim Biophys Acta Mol Basis Dis. 2020 Mar 1;1866(3):165620. doi: 10.1016/j.bbadis.2019.165620. Epub 2019 Nov 25. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31778749 Free PMC article. - Modulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): lessons from genetically modified mice.
Massion PB, Balligand JL. Massion PB, et al. J Physiol. 2003 Jan 1;546(Pt 1):63-75. doi: 10.1113/jphysiol.2002.025973. J Physiol. 2003. PMID: 12509479 Free PMC article. Review. - Effects of arginase inhibition on myocardial Ca2+ and contractile responses.
Cho JS, Han YS, Jensen C, Sieck G. Cho JS, et al. Physiol Rep. 2022 Jul;10(14):e15396. doi: 10.14814/phy2.15396. Physiol Rep. 2022. PMID: 35866269 Free PMC article. - Modulatory Role of Nitric Oxide/cGMP System in Endothelin-1-Induced Signaling Responses in Vascular Smooth Muscle Cells.
Kapakos G, Bouallegue A, Daou GB, Srivastava AK. Kapakos G, et al. Curr Cardiol Rev. 2010 Nov;6(4):247-54. doi: 10.2174/157340310793566055. Curr Cardiol Rev. 2010. PMID: 22043200 Free PMC article. - Nitric oxide effects depend on different mechanisms in different regions of the rat heart.
Derici K, Samsar U, Demirel-Yilmaz E. Derici K, et al. Heart Vessels. 2012 Jan;27(1):89-97. doi: 10.1007/s00380-011-0116-6. Epub 2011 Feb 11. Heart Vessels. 2012. PMID: 21311889
References
- Al-Hillawi E, Chilton D, Trayer IP, Cummins P. Phosphorylation-specific antibodies for human cardiac troponin-I. European Journal of Biochemistry. 1998;256:535–540. - PubMed
- Blumenthal DK, Stull JT, Gill GN. Phosphorylation of cardiac troponin by guanosine 3′,5′-monophosphate-dependent protein kinase. Journal of Biological Chemistry. 1978;253:334–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous