Vago-sympathoadrenal reflex in thermogenesis induced by osmotic stimulation of the intestines in the rat - PubMed (original) (raw)
Vago-sympathoadrenal reflex in thermogenesis induced by osmotic stimulation of the intestines in the rat
Toshimasa Osaka et al. J Physiol. 2002.
Abstract
Duodenal infusion of hypertonic solutions elicits osmolality-dependent thermogenesis in urethane-anaesthetized rats. Here we investigated the involvement of the autonomic nervous system, adrenal medulla and brain in the mechanism of this thermogenesis. Bilateral subdiaphragmatic vagotomy greatly attenuated the first hour, but not the later phase, of the thermogenesis induced by 3.6 % NaCl (10 ml kg(-1)). Neither atropine pretreatment (10 mg kg(-1), I.P.) nor capsaicin desensitization had any effect on the osmotically induced thermogenesis, suggesting the involvement of non-nociceptive vagal afferents. Bilateral splanchnic denervation caudal to the suprarenal ganglia also had no effect, suggesting a lack of involvement of spinal afferents and sympathetic efferents to the major upper abdominal organs. Adrenal demedullation greatly attenuated the initial phase, but not the later phase, of thermogenesis. Pretreatment with the beta-blocker propranolol (20 mg kg(-1), I.P.) attenuated the thermogenesis throughout the 3 h observation period. The plasma adrenaline concentration increased significantly 20 min after osmotic stimulation but returned to the basal level after 60 min. The plasma noradrenaline concentration increased 20 min after osmotic stimulation and remained significantly elevated for 120 min. Therefore, adrenaline largely mediated the initial phase of thermogenesis, and noradrenaline was involved in the entire thermogenic response. Moreover, neither decerebration nor pretreatment with the antipyretic indomethacin (10 mg kg(-1), S.C.) had any effect. Accordingly, this thermogenesis did not require the forebrain and was different from that associated with fever. These results show the critical involvement of the vagal afferents, hindbrain and sympathoadrenal system in the thermogenesis induced by osmotic stimulation of the intestines.
Figures
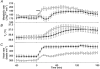
Figure 1. Effects of denervation of vagus or splanchnic nerves, pretreatment with atropine, or capsaicin desensitization on responses to duodenal infusion of hypertonic NaCl
Metabolic rate (A) and _T_c (B) following vagotomy (•, n = 4), splanchnic denervation (▾, n = 4), atropine pretreatment (○, n = 4) and capsaicin desensitization (▿, n = 4). Hypertonic (3.6 %) NaCl was infused into the duodenum at a volume of 10 ml kg−1 for 10 min. The horizontal bar shows the period of NaCl infusion. The metabolic rate of vagotomized rats was significantly lower than other rats at 25–30 min, and the energy expenditure during the first hour was significantly lower in vagotomized rats than in the other groups, although energy expenditure during the 1–3 h period was comparable among all groups. Values are means and vertical bars show
s.e.m.
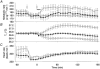
Figure 2. Effects of adrenal demedullation on duodenal hypertonic NaCl-induced responses
The metabolic rate (A), _T_c (B), and heart rate (C)of adrenal-demedullated rats (•, n = 6) and sham-operated control rats (○, n = 5). The metabolic rate of adrenal-medullated rats was significantly lower than that of sham-operated control rats for 20–50 min following infusion of 3.6 % NaCl, and the initial energy expenditure during the first hour in adrenal-demedullated rats was significantly lower than in control rats, although energy expenditure during the 1–3 h period was comparable. The horizontal bar shows the period of NaCl infusion.
Figure 3. Contribution of β-adrenoceptors to the osmotically induced thermogenesis
The effect on metabolic rate (A), _T_c (B) and heart rate (C) of duodenal infusion of 3.6 % NaCl in propranolol-treated (•, n = 5) and control (▵, n = 5) rats, and 0.9 % NaCl infusion in propranolol-treated rats (○, n = 5). The propranolol-treated rats exhibited a significantly smaller thermogenic response than the control rats. However, propranolol-treated rats expended energy to a significantly higher degree after the 3.6 % NaCl infusion than after the 0.9 % NaCl infusion. Reflecting the changes in metabolic rate, _T_c decreased in propanolol-treated rats after infusion of 0.9 % NaCl, but not after infusion of 3.6 % NaCl, and increased in control rats that had been infused with 3.6 % NaCl without administration of propranolol. Administration of propranolol alone significantly decreased the basal heart rate, and the changes in heart rate were similar between rats infused with 0.9 % NaCl and 3.6 % NaCl. We did not record the heart rate of the control group. The arrows show the time of propranolol administration; the horizontal bar shows the period of NaCl infusion.
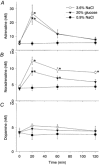
Figure 4. Changes in plasma levels of noradrenaline, adrenaline and dopamine after infusion of 3.6 % NaCl, 20 % glucose or 0.9 % NaCl
Changes in plasma levels of noradrenaline (A), adrenaline (B), and dopamine (C) after infusion of 3.6 % NaCl (○, n = 6), 20 % glucose (▾, n = 6) or 0.9 % NaCl (•, n = 5). A, infusion of 3.6 % NaCl or 20 % glucose, but not 0.9 % NaCl, markedly increased the plasma adrenaline level at 20 min, although the level rapidly returned to basal. B, the plasma noradrenaline level increased after the infusion of either hypertonic solution, and the significant increase lasted for 2 h in the rats infused with 3.6 % NaCl, and for 1 h in the rats infused with 20 % glucose. C, the infusion of neither 3.6 % NaCl nor 20 % glucose had any effect on the plasma level of dopamine. * Significant difference from the corresponding value for the group infused with 0.9 % NaCl.
Similar articles
- Thermogenesis induced by intravenous infusion of hypertonic solutions in the rat.
Kobayashi A, Osaka T, Inoue S, Kimura S. Kobayashi A, et al. J Physiol. 2001 Sep 1;535(Pt 2):601-10. doi: 10.1111/j.1469-7793.2001.00601.x. J Physiol. 2001. PMID: 11533148 Free PMC article. - Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system.
Osaka T, Endo M, Yamakawa M, Inoue S. Osaka T, et al. Peptides. 2005 Sep;26(9):1623-31. doi: 10.1016/j.peptides.2005.02.016. Epub 2005 Mar 17. Peptides. 2005. PMID: 16112402 - Thermogenesis induced by osmotic stimulation of the intestines in the rat.
Osaka T, Kobayashi A, Inoue S. Osaka T, et al. J Physiol. 2001 Apr 1;532(Pt 1):261-9. doi: 10.1111/j.1469-7793.2001.0261g.x. J Physiol. 2001. PMID: 11283240 Free PMC article. - Effects of nutrient intake on sympathoadrenal activity and thermogenic mechanisms.
Astrup AV, Christensen NJ, Simonsen L, Bülow J. Astrup AV, et al. J Neurosci Methods. 1990 Sep;34(1-3):187-92. doi: 10.1016/0165-0270(90)90057-m. J Neurosci Methods. 1990. PMID: 2259241 Review. - Sympathoadrenal system and regulation of thermogenesis.
Landsberg L, Saville ME, Young JB. Landsberg L, et al. Am J Physiol. 1984 Aug;247(2 Pt 1):E181-9. doi: 10.1152/ajpendo.1984.247.2.E181. Am J Physiol. 1984. PMID: 6380306 Review.
Cited by
- The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not.
Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. Romanovsky AA, et al. Pharmacol Rev. 2009 Sep;61(3):228-61. doi: 10.1124/pr.109.001263. Epub 2009 Sep 11. Pharmacol Rev. 2009. PMID: 19749171 Free PMC article. Review. - Thermogenesis elicited by skin cooling in anaesthetized rats: lack of contribution of the cerebral cortex.
Osaka T. Osaka T. J Physiol. 2004 Mar 1;555(Pt 2):503-13. doi: 10.1113/jphysiol.2003.053215. Epub 2003 Oct 24. J Physiol. 2004. PMID: 14578483 Free PMC article. - The influence of oral water load on energy expenditure and sympatho-vagal balance in obese and normal weight women.
Kocełak P, Zak-Gołąb A, Rzemieniuk A, Smętek J, Sordyl R, Tyrka A, Sosnowski M, Zahorska-Markiewicz B, Chudek J, Olszanecka-Glinianowicz M. Kocełak P, et al. Arch Med Sci. 2012 Dec 20;8(6):1003-8. doi: 10.5114/aoms.2012.32406. Epub 2012 Dec 19. Arch Med Sci. 2012. PMID: 23319974 Free PMC article. - Electrophysiological analysis of the mechanism of autonomic action by lactobacilli.
Tanida M, Nagai K. Tanida M, et al. Biosci Microflora. 2011;30(4):99-109. doi: 10.12938/bifidus.30.99. Epub 2011 Nov 17. Biosci Microflora. 2011. PMID: 25045315 Free PMC article. Review. - Role of diabetes, hypertension, and cigarette smoking on atherosclerosis.
Mathur RK. Mathur RK. J Cardiovasc Dis Res. 2010 Apr;1(2):64-8. doi: 10.4103/0975-3583.64436. J Cardiovasc Dis Res. 2010. PMID: 20877688 Free PMC article.
References
- Berthoud HR, Jeanrenaud B. Sham feeding-induced cephalic phase insulin release in the rat. American Journal of Physiology. 1982;242:E280–285. - PubMed
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. Journal of the Autonomic Nervous System. 1990;31:191–201. - PubMed
- Choi-Kwon S, Baertschi AJ. Splanchnic osmosensation and vasopressin: mechanisms and neural pathways. American Journal of Physiology. 1991;261:E18–25. - PubMed
- Gõbel Gy, Ember Á, Pétervári E, Kis A, Székely M. Postalimentary hyperthermia: a role for gastrointestinal but not for caloric signals. Journal of Thermal Biology. 2001;26:519–523.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources