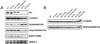Tau is essential to beta -amyloid-induced neurotoxicity - PubMed (original) (raw)
Tau is essential to beta -amyloid-induced neurotoxicity
Mark Rapoport et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Senile plaques and neurofibrillary tangles, the two hallmark lesions of Alzheimer's disease, are the results of the pathological deposition of proteins normally present throughout the brain. Senile plaques are extracellular deposits of fibrillar beta-amyloid peptide (Abeta); neurofibrillary tangles represent intracellular bundles of self-assembled hyperphosphorylated tau proteins. Although these two lesions are often present in the same brain areas, a mechanistic link between them has yet to be established. In the present study, we analyzed whether tau plays a key role in fibrillar Abeta-induced neurite degeneration in central neurons. Cultured hippocampal neurons obtained from wild-type, tau knockout, and human tau transgenic mice were treated with fibrillar Abeta. Morphological analysis indicated that neurons expressing either mouse or human tau proteins degenerated in the presence of Abeta. On the other hand, tau-depleted neurons showed no signs of degeneration in the presence of Abeta. These results provide direct evidence supporting a key role for tau in the mechanisms leading to Abeta-induced neurodegeneration in the central nervous system. In addition, the analysis of the composition of the cytoskeleton of tau-depleted neurons suggested that the formation of more dynamic microtubules might confer resistance to Abeta-mediated neurodegeneration.
Figures
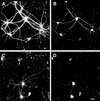
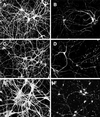
Figure 1
Fibrillar Aβ induced neurite degeneration in mature hippocampal neurons. Hippocampal neurons obtained from wild-type E16 embryos were kept in culture for 4 weeks (A and B) and then incubated in the presence of fibrillar Aβ for 4 days (C and D). Cells were fixed and double stained with tubulin (polyclonal antitubulin, Sigma) (A and C) and tau (clone tau-5) (B and D) antibodies. Note the massive neurite degeneration induced by fibrillar Aβ (C and D). (Bar = 20 μm.)
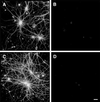
Figure 2
Tau-depleted hippocampal neurons did not degenerate in the presence of fibrillar Aβ. Hippocampal neurons obtained from tau knockout E16 embryos were kept in culture for 4 weeks (A and B) and then incubated in the presence of fibrillar Aβ for 4 days (C and D). Cells were fixed and double stained with tubulin (polyclonal antitubulin, Sigma) (A and C) and tau (clone tau-5) (B and D) antibodies. Most of the processes extended by tau knockout neurons showed normal morphological characteristic in the presence of fibrillar Aβ (C). (Bar = 20 μm.)
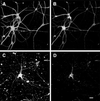
Figure 3
Cultured hippocampal neurons expressing human tau degenerated in the presence of fibrillar Aβ. Hippocampal neurons obtained from human tau transgenic mice were kept in culture for 4 weeks and then incubated in the presence (C and D) or absence (A and B) of fibrillar Aβ for 4 days. Cells were fixed and double stained with tubulin (polyclonal antitubulin, Sigma) (A and C) and tau (clone tau-5) (B and D) antibodies. (Bar = 20 μm.)
Figure 4
(A) Western blot analysis of the content of microtubular proteins in wild-type (wt), human tau transgenic (htau transg), and knockout (KO) tau hippocampal neurons kept in culture for 4 weeks and incubated in the presence or absence of fibrillar Aβ for 4 days. Equal amounts of total protein (40 μg) were loaded in each lane. (B) Western blot analysis of the content of α-tubulin and detyrosinated tubulin in cytoskeletal fractions prepared from wild-type (wt), human tau transgenic (htau transg), and knockout (KO) tau hippocampal neurons kept in culture for 4 weeks and incubated in the presence or absence of nocodazole or Taxol. Nocodazole-resistant fractions were normalized using the content of α-tubulin as internal controls.
Figure 5
Fibrillar Aβ induced neurite degeneration in Taxol-treated tau-depleted hippocampal neurons. Wild-type (A and B), tau knockout (C and D), and human tau transgenic (E and F) hippocampal neurons kept in culture for 4 weeks were pretreated with Taxol for 6 h and then cultured for 24 h in the presence (B, D, and F) or absence (A, C, and E) of fibrillar Aβ. Cells were then fixed and stained by using a tubulin antibody (clone DMIA, SIGMA). Severe neurodegeneration was detected in Taxol-treated tau knockout hippocampal neurons exposed to fibrillar Aβ (D) as well as in neurons expressing either murine (B) or human (F) tau isoforms. (Bar = 20 μm.)
Similar articles
- Soluble Conformers of Aβ and Tau Alter Selective Proteins Governing Axonal Transport.
Sherman MA, LaCroix M, Amar F, Larson ME, Forster C, Aguzzi A, Bennett DA, Ramsden M, Lesné SE. Sherman MA, et al. J Neurosci. 2016 Sep 14;36(37):9647-58. doi: 10.1523/JNEUROSCI.1899-16.2016. J Neurosci. 2016. PMID: 27629715 Free PMC article. - Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.
Torres AK, Jara C, Park-Kang HS, Polanco CM, Tapia D, Alarcón F, de la Peña A, Llanquinao J, Vargas-Mardones G, Indo JA, Inestrosa NC, Tapia-Rojas C. Torres AK, et al. J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review. - Aβ-induced acceleration of Alzheimer-related τ-pathology spreading and its association with prion protein.
Gomes LA, Hipp SA, Rijal Upadhaya A, Balakrishnan K, Ospitalieri S, Koper MJ, Largo-Barrientos P, Uytterhoeven V, Reichwald J, Rabe S, Vandenberghe R, von Arnim CAF, Tousseyn T, Feederle R, Giudici C, Willem M, Staufenbiel M, Thal DR. Gomes LA, et al. Acta Neuropathol. 2019 Dec;138(6):913-941. doi: 10.1007/s00401-019-02053-5. Epub 2019 Aug 14. Acta Neuropathol. 2019. PMID: 31414210 - Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures.
Zheng WH, Bastianetto S, Mennicken F, Ma W, Kar S. Zheng WH, et al. Neuroscience. 2002;115(1):201-11. doi: 10.1016/s0306-4522(02)00404-9. Neuroscience. 2002. PMID: 12401334 - [The lesions of Alzheimer's disease: which therapeutic perspectives?].
Duyckaerts C, Perruchini C, Lebouvier T, Potier MC. Duyckaerts C, et al. Bull Acad Natl Med. 2008 Feb;192(2):303-18; discussion 318-21. Bull Acad Natl Med. 2008. PMID: 18819685 Review. French.
Cited by
- Tau and neuron aging.
Avila J, de Barreda EG, Pallas-Bazarra N, Hernandez F. Avila J, et al. Aging Dis. 2013 Feb;4(1):23-8. Epub 2012 Dec 3. Aging Dis. 2013. PMID: 23423462 Free PMC article. - Perivascular spaces as a potential biomarker of Alzheimer's disease.
Lynch M, Pham W, Sinclair B, O'Brien TJ, Law M, Vivash L. Lynch M, et al. Front Neurosci. 2022 Oct 18;16:1021131. doi: 10.3389/fnins.2022.1021131. eCollection 2022. Front Neurosci. 2022. PMID: 36330347 Free PMC article. Review. - "Untangling" Alzheimer's disease and epilepsy.
Scharfman HE. Scharfman HE. Epilepsy Curr. 2012 Sep;12(5):178-83. doi: 10.5698/1535-7511-12.5.178. Epilepsy Curr. 2012. PMID: 23118602 Free PMC article. - Calpain dysregulation in Alzheimer's disease.
Ferreira A. Ferreira A. ISRN Biochem. 2012 Oct 16;2012:728571. doi: 10.5402/2012/728571. eCollection 2012. ISRN Biochem. 2012. PMID: 25969760 Free PMC article. Review. - Spatiotemporal Correlation between Amyloid and Tau Accumulations Underlies Cognitive Changes in Aging.
Kim CM, Diez I, Bueichekú E, Ahn S, Montal V, Sepulcre J. Kim CM, et al. J Neurosci. 2024 Feb 14;44(7):e0488232023. doi: 10.1523/JNEUROSCI.0488-23.2023. J Neurosci. 2024. PMID: 38123362 Free PMC article.
References
- Glenner G G, Wong C W. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
- Vitek M P, Rasool C G, de Sauvage F, Vitek S M, Bartus R T, Beer B, Ashton R A, Macq A F, Maloteaux J M, Blume A J. Brain Res. 1988;464:121–131. - PubMed
- Yankner B A, Mesulam M M. N Engl J Med. 1991;325:1849–1857. - PubMed
- Haass C, Selkoe D J. Cell. 1993;75:1039–1042. - PubMed
- Selkoe D J. Annu Rev Cell Biol. 1994;10:373–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases