T-bet regulates IgG class switching and pathogenic autoantibody production - PubMed (original) (raw)
T-bet regulates IgG class switching and pathogenic autoantibody production
Stanford L Peng et al. Proc Natl Acad Sci U S A. 2002.
Abstract
A molecular understanding of the regulation of IgG class switching to IL-4-independent isotypes, particularly to IgG2a, remains largely unknown. The T-box transcription factor T-bet directly regulates Th1 lineage commitment by CD4 T cells, but its role in B lymphocytes has been largely unexplored. We show here a role for T-bet in the regulation of IgG class switching, especially to IgG2a. T-bet-deficient B lymphocytes demonstrate impaired production of IgG2a, IgG2b, and IgG3 and, most strikingly, are unable to generate germ-line or postswitch IgG2a transcripts in response to IFN-gamma. Conversely, enforced expression of T-bet initiates IgG2a switching in cell lines and primary cells. This function contributes critically to the pathogenesis of murine lupus, where the absence of T-bet strikingly reduces B cell-dependent manifestations, including autoantibody production, hypergammaglobulinemia, and immune-complex renal disease and, in particular, abrogates IFN-gamma-mediated IgG2a production. Classical T cell manifestations persisted, including lymphadenopathy and cellular infiltrates of skin and liver. These results identify T-bet as a selective transducer of IFN-gamma-mediated IgG2a class switching in B cells and emphasize the importance of this regulation in the pathogenesis of humorally mediated autoimmunity.
Figures
Figure 1
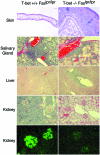
Role of T-bet in the autoimmune histopathology in lupus-prone mice. Indicated tissues from 12-week-old T-bet+/+Faslpr/lpr or T-bet−/−Faslpr/lpr animals were fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin. For the bottom images, kidneys from respective mice were embedded frozen in OCT medium, sectioned, and stained with FITC–anti-mouse IgG antibody. Representative sections from eight animals examined from each genotype are shown. Clockwise from Upper Left, bar corresponds to 100 μm, 100 μm, 50 μm, 100 μm, 25 μm, 25 μm, 25 μm, 25 μm, 100 μm, and 50 μm.
Figure 2
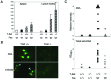
Role of T-bet in serological and lymphoid manifestations of systemic autoimmunity. (A) Lymphoid organomegaly was assayed in 12-week-old animals of the indicated genotypes by total cell counts (in millions) of erythrocyte-depleted spleen and lymph nodes (n = 4–6 in each group). (B and_C_) Sera from 12-week-old animals of the indicated genotypes were assayed by the fluorescent antinuclear antibody test on Hep-2 substrates at 1:50 dilution and, for anti-DNA antibodies, by ELISA and Crithidia immunofluorescence. Yellow arrow indicates the Crithidia nucleus, where fluorescent staining indicates the presence of antinuclear antibody activity; blue arrow indicates the doubled-stranded DNA (dsDNA)-containing kinetoplast, where fluorescent staining specifically indicates the presence of anti-dsDNA activity. Some sera from T-bet−/−Faslpr/lpr animals displayed modest, generalized reactivity against DNA in a plate-bound ELISA assay, but all T-bet−/−Faslpr/lpr sera were negative for anti-dsDNA autoantibody activity as assayed by_Crithidia_. (n = 8–10 in each group.)
Figure 3
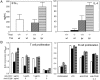
T-bet is partially dispensable for cytokine production. (A) Cytokine production by T cells. Naive CD4+ T cells were purified from spleen and lymph nodes by negative selection (R & D Systems) and stimulated for 72 h in RPMI/10% with 1 μg/ml anti-murine CD28 (37.51) antibody and 1 μg/ml plate-bound anti-murine CD3 (145–2C11) antibody (PharMingen). Cytokine production was evaluated in culture supernatants by ELISA (PharMingen) from three to five 12-week-old animals of each genotype. (B) T and B cell proliferation. T and B cell proliferation was measured by BrdUrd incorporation (Amersham Pharmacia) on purified naive CD4 T cells or purified resting B cells, derived from three to five 12-week-old animals of each genotype, by using the stimulatory conditions indicated. BrdUrd incorporation was measured by pulsing cells at 48 h of stimulation, followed by assay at 72 h. *, Undetectable by assay (<2 ng/ml). Open bars, T-bet+/+Faswt/wt; solid bars, T-bet−/−Faswt/wt; diagonally hatched bars, T-bet+/+Faslpr/lpr; horizontally hatched bars, T-bet−/−Faslpr/lpr.
Figure 4
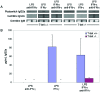
Ig production in T-bet-deficient mice. (A and_B_) Serum Ig isotype titers were evaluated on 12-week-old Faswt/wt (A) and Faslpr/lpr(B) animals of the indicated T-bet genotypes by ELISA. (C and D) In vitro class switching was assayed in 48-h culture supernatants from purified CD43-depleted B cells. Three Faswt/wt (C) and Faslpr/lpr (D) animals of indicated T-bet genotypes were assayed for Ig isotype production by ELISA. Stimulatory conditions included: IgM, IgA, IgG2b, and IgG3, LPS 25 μg/ml; IgG1 and IgE, anti-CD40 2 μg/ml + 10 ng/ml recombinant mouse (rm) IL-4; IgG2a, LPS 25 μg/ml + 100 ng/ml rmIFN-γ. *, Undetectable by assay (less than 20 pg/ml).
Figure 5
T-bet is required for IgG2a isotype switching by resting B cells. (A) CSR transcripts in MRL B cells. Germ-line and postswitch class-switching transcripts were assayed by RT-PCR on total RNA purified from CD43-depleted Fas+ B cells stimulated for 96 h in RPMI/10% supplemented with 25 μg/ml LPS ± rmIFN-γ at the concentrations indicated, as described (21). (B) CSR transcripts in F2(129 × B6) B cells. Germ-line and postswitch class-switching transcripts were assayed by RT-PCR on total RNA purified from CD43-depleted B cells stimulated for 96 h in RPMI/10% supplemented with 25 μg/ml LPS ± rmIFN-γ at the concentrations indicated.
Figure 6
T-bet-dependent regulation of IgG2a transcription. (A) 18.81 murine pre-B cells were transfected with a T-bet or control expression vector and assayed for the presence of germ-line IgG2a transcripts by RT-PCR on total RNA after 6 h in RPMI/10% supplemented with 100 ng/ml rmIFN-γ or 10 μg/ml neutralizing anti-IFN-γ antibody. (B) Purified CD43-depleted B cells from T-bet-deficient Faswt/wt mice were stimulated by 25 μg/ml LPS and 100 ng/ml rmIFN-γ for 24 h, transduced by a T-bet-GFP or control-GFP retrovirus, and assayed for the presence of germ-line IgG2a transcripts by RT-PCR on total RNA after 24 h of further incubation in medium containing 25 μg/ml LPS and 100 ng/ml rmIFN-γ. (C) Purified CD43-depleted B cells from T-bet+/+Faslpr/lprand T-bet−/−Faslpr/lpr mice were stimulated by 25 μg/ml LPS for 24 h and transduced by a T-bet-GFP or control-GFP retrovirus, and the culture supernatant was assayed for secreted IgG2a after a further 48 h in medium containing 25 μg/ml LPS. (D) Class-switch assays were performed on purified CD43-depleted B cells from IFN-γR-deficient animals bearing a transgene that enforces T-bet expression under the control of the CMV promoter or IFN-γR-deficient, nontransgenic littermates. All cells were stimulated with 25 μg/ml LPS, supplemented with 10 ng/ml rmIL-4 for IgG1 and 100 ng/ml rmIFN-γ for IgG2a. Results from three animals in each group are shown; a BALB/c animal was used for comparison. (E) The ability of T-bet to transactivate germ-line IgG2a and IgE promoter–firefly luciferase constructs was tested in transient transfection assays in M12 cells. Cells were electroporated simultaneously with 10 μg of reporter plasmid, 10 μg of either control (pCMV) or T-bet-expressing (pCMV-T-bet) plasmids, and 0.2 μg of a pCMV-Renilla luciferase control expression plasmid. Firefly luciferase luminescence was determined upon whole-cell extracts within 6 h, normalized for Renilla luciferase. For IgG2a, cells were treated with 100 ng/ml rmIFN-γ; for IgE, cells were treated with 2 μg/ml anti-CD40 and 10 ng/ml rmIL-4. Ig promoter reporter constructs were based on the firefly luciferase reporter vector pGL3-Basic (Promega). ɛ-Luciferase was generated by deleting the HS1,2 sites from GLɛ promoter-HS1,2-luc (22). γ2a-Luciferase was generated by replacing the _Hin_dIII-_Bgl_II promoter fragment of ɛ-luciferase with an ≈773-bp_Pst_I-_Pst_I genomic fragment from plasmid E1.6, corresponding to approximately −520/+253 with regard to the presumed first transcription start site of the Iγ2a exon (23). Electroporation was performed by using 10–20 μg of supercoiled plasmid, 5 × 106 cells, 0.4-cm cuvette, at 280 V, 975 μF in a Gene Pulser II (Bio-Rad). Luciferase activity was determined by using the Dual-Luciferase Reporter Assay System (Promega). Shown is a single experiment, representative of three.
Figure 7
IFN-α can induce IgG2a in a T-bet-independent fashion. Germ-line and postswitch IgG2a transcripts were assayed by RT-PCR (A) and IgG2a levels were measured by ELISA (B) on culture supernatant of purified CD43-depleted B cells from T-bet+/+Faswt/wt and T-bet−/−Faswt/wt mice stimulated by 25 μg/ml LPS for 96 h, supplemented with 100 ng/ml rmIFN-γ, 10 μg/ml neutralizing anti-IFN-γ, and/or 100 units/ml rmIFN-α as indicated. In A, duplicate studies performed simultaneously are shown. In B, data represent three animals in each group. *, undetectable by assay (<20 pg/ml).
Similar articles
- T-bet regulates T-independent IgG2a class switching.
Gerth AJ, Lin L, Peng SL. Gerth AJ, et al. Int Immunol. 2003 Aug;15(8):937-44. doi: 10.1093/intimm/dxg093. Int Immunol. 2003. PMID: 12882831 - Induction of IgG2a class switching in B cells by IL-27.
Yoshimoto T, Okada K, Morishima N, Kamiya S, Owaki T, Asakawa M, Iwakura Y, Fukai F, Mizuguchi J. Yoshimoto T, et al. J Immunol. 2004 Aug 15;173(4):2479-85. doi: 10.4049/jimmunol.173.4.2479. J Immunol. 2004. PMID: 15294962 - Stat1-dependent synergistic activation of T-bet for IgG2a production during early stage of B cell activation.
Xu W, Zhang JJ. Xu W, et al. J Immunol. 2005 Dec 1;175(11):7419-24. doi: 10.4049/jimmunol.175.11.7419. J Immunol. 2005. PMID: 16301649 - T cells in murine lupus: propagation and regulation of disease.
Peng SL, Craft J. Peng SL, et al. Mol Biol Rep. 1996;23(3-4):247-51. doi: 10.1007/BF00351176. Mol Biol Rep. 1996. PMID: 9112236 Review. - Mechanisms of mouse T lymphocyte-induced suppression of the IgG2ab allotype and T lymphocyte tolerance to IgG2ab.
Majlessi L, Bordenave G. Majlessi L, et al. Arch Immunol Ther Exp (Warsz). 2001;49(6):407-15. Arch Immunol Ther Exp (Warsz). 2001. PMID: 11814234 Review.
Cited by
- Immunometabolic effects of lactate on humoral immunity in healthy individuals of different ages.
Romero M, Miller K, Gelsomini A, Garcia D, Li K, Suresh D, Frasca D. Romero M, et al. Nat Commun. 2024 Aug 30;15(1):7515. doi: 10.1038/s41467-024-51207-x. Nat Commun. 2024. PMID: 39209820 Free PMC article. - Bank1 modulates the differentiation and molecular profile of key B cell populations in autoimmunity.
Gómez Hernández G, Domínguez T, Galicia G, Morell M, Alarcón-Riquelme ME. Gómez Hernández G, et al. JCI Insight. 2024 Aug 20;9(19):e179417. doi: 10.1172/jci.insight.179417. JCI Insight. 2024. PMID: 39163122 Free PMC article. - A TNIP1-driven systemic autoimmune disorder with elevated IgG4.
Medhavy A, Athanasopoulos V, Bassett K, He Y, Stanley M, Enosi Tuipulotu D, Cappello J, Brown GJ, Gonzalez-Figueroa P, Turnbull C, Shanmuganandam S, Tummala P, Hart G, Lea-Henry T, Wang H, Nambadan S, Shen Q, Roco JA, Burgio G, Wu P, Cho E, Andrews TD, Field MA, Wu X, Ding H, Guo Q, Shen N, Man SM, Jiang SH, Cook MC, Vinuesa CG. Medhavy A, et al. Nat Immunol. 2024 Sep;25(9):1678-1691. doi: 10.1038/s41590-024-01902-0. Epub 2024 Jul 26. Nat Immunol. 2024. PMID: 39060650 Free PMC article. - Hem1 inborn errors of immunity: waving goodbye to coordinated immunity in mice and humans.
Christodoulou A, Tsai JY, Suwankitwat N, Anderson A, Iritani BM. Christodoulou A, et al. Front Immunol. 2024 Jul 4;15:1402139. doi: 10.3389/fimmu.2024.1402139. eCollection 2024. Front Immunol. 2024. PMID: 39026677 Free PMC article. Review. - Autoimmune diseases and atherosclerotic cardiovascular disease.
Porsch F, Binder CJ. Porsch F, et al. Nat Rev Cardiol. 2024 Nov;21(11):780-807. doi: 10.1038/s41569-024-01045-7. Epub 2024 Jun 27. Nat Rev Cardiol. 2024. PMID: 38937626 Review.
References
- Snapper C M, Finkelman F D. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott; 1999. pp. 831–861.
- Bacharier L B, Geha R S. J Allergy Clin Immunol. 2000;105:S547–S558. - PubMed
- Snapper C, Marcu K B, Zelazowski P. Immunity. 1997;6:217–223. - PubMed
- Snapper C M, Paul W E. Science. 1987;236:944–947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials