Cooperative, ATP-dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters - PubMed (original) (raw)
Cooperative, ATP-dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters
Jonathan E Moody et al. J Biol Chem. 2002.
Abstract
ATP-binding cassette (ABC) transporters harvest the energy present in cellular ATP to drive the translocation of a structurally diverse set of solutes across the membrane barriers of eubacteria, archaebacteria, and eukaryotes. The positively cooperative ATPase activity (Hill coefficient, 1.7) of a model soluble cassette of known structure, MJ0796, from Methanococcus jannaschii indicates that at least two binding sites participate in the catalytic reaction. Mutation of the catalytic base in MJ0796, E171Q, produced a cassette that can bind but not efficiently hydrolyze ATP. The equivalent mutation (E179Q) in a homologous cassette, MJ1267, had an identical effect. Both mutant cassettes formed dimers in the presence of ATP but not ADP, indicating that the energy of ATP binding is first coupled to the transport cycle through a domain association reaction. The non-hydrolyzable nucleotides adenosine 5'-(beta,gamma-imino)triphosphate and adenosine 5'-3-O-(thio)triphosphate were poor analogues of ATP in terms of their ability to promote dimerization. Moreover, inclusion of MgCl2, substitution of KCl for NaCl, or alterations in the polarity of the side chain at the catalytic base all weakened the ATP-dependent dimer, suggesting that electrostatic interactions are critical for the association reaction. Thus, upon hydrolysis of bound ATP and the release of product, both electrostatic and conformational changes drive the cassettes apart, providing a second opportunity to couple free energy changes to the transport reaction.
Figures
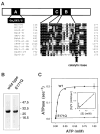
Fig. 1. Mutation of the catalytic base in the ABC ATPase cassettes
A, consensus sequences in the ATP-binding cassettes of ABC transporters. An alignment of the Walker B and transporter consensus (LSGGQ) regions of several transporters including those of known three-dimensional structure is shown. The Glu (E) (arrow) in the DEP sequence at the end of the Walker B sequence is in position to serve as the catalytic base. B, Coomassie Blue-stained 10% SDS-polyacrylamide gel of wild type MJ0796 and the E171Q mutant. Positions of protein marker bands are illustrated by arrows. C, wild type MJ0796 exhibited cooperative ATPase activity, whereas the mutant E171Q exhibited no measurable activity at ATP levels as high as 5 mM (data not shown). A _V_max of 0.2 s−1, a Km of 50 _μ_M, and a Hill constant of 1.7 were used to produce the fit (solid line). A Hanes-Woolf plot of the data (inset) shows positive cooperativity. WT, wild type.
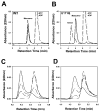
Fig. 2. Analytical gel filtration assays of nucleotide-dependent cassette dimerization
Wild type (A) or E171Q (B) MJ0796 were analyzed at a 30 _μ_M concentration without nucleotide (——), with 10 mM ATP (— —), or with 10 mM ADP (· · · ·). Column calibration standards are labeled: a, bovine serum albumin (66 kDa); b, carbonic anhydrase (34 kDa); c, lysozyme (14 kDa). Assays were conducted on both the E171Q mutant of MJ0796 (5 _μ_M) in the presence of 0 _μ_M (— · · —), 5 _μ_M (— —), 10 _μ_M (— · —), 20 _μ_M (· · · ·), or 200 _μ_M (——) ATP (C) and the equivalent E179Q mutant of MJ1267 (30 _μ_M) in the presence of 0 _μ_M (— · · —), 25 _μ_M (— —), 50 _μ_M (— · —), 500 _μ_M (· · · ·), or 1 mM (——) ATP (D). WT, wild type.
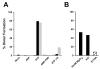
Fig. 3. Effect of non-hydrolyzable nucleotide analogues and cations on dimerization of MJ0796-E171Q and MJ1267-E179Q
The percentage of dimer in gel filtration assays was calculated based on measurement of peak heights. Protein concentration was 30 _μ_M. A, ADP, ATP, AMP-PNP, and ATP_γ_S were assayed at 10 mM for their ability to form MJ0796-WT (open bars), MJ0796-E171Q (black bars), or MJ1267-E179Q (gray bars) dimers in a buffer containing 200 mM NaCl, 50 mM Tris-Cl, pH 7.6. B, the effects of changes in the salt environment during MJ0796-E179Q dimer formation and column chromatography were evaluated in the presence of 10 mM ATP. Either 10 mM MgCl2 was added to the buffer or 200 mM KCl was substituted for the 200 mM NaCl. The E179A mutant of MJ1267 was also evaluated in the standard NaCl buffer (hatched bar).
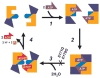
Fig. 4. A model for the reaction cycle of ABC transporters based on ATP-dependent dimerization of ATP-binding cassettes
The ATP-binding core and antiparallel _β_-subdomain are shown in yellow. The initial step (step 1) combines binding of ATP to protein monomers with the _α_-helical subdomain (dark blue) rotation and _γ_-phosphate linker (light blue) shift. This step is rapidly followed by cassette dimerization (step 2). Hydrolysis of ATP (step 3) results in the reversal of the previous conformational changes coupled to dissociation of the dimer and release of hydrolysis products (step 4). The E171Q mutant of MJ0796 and the equivalent E179Q mutant of MJ1267 become locked in the dimeric state because hydrolysis cannot occur, and the protein remains in the thermodynamically favorable ATP-bound conformation. The cooperativity of ATP hydrolysis exhibited by wild type MJ0796 (Hill constant of 1.7) supports this model as the dimer formation occurs concomitantly with the cooperative binding of two ATP molecules.
Similar articles
- ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer.
Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. Smith PC, et al. Mol Cell. 2002 Jul;10(1):139-49. doi: 10.1016/s1097-2765(02)00576-2. Mol Cell. 2002. PMID: 12150914 Free PMC article. - Crystal structures of the MJ1267 ATP binding cassette reveal an induced-fit effect at the ATPase active site of an ABC transporter.
Karpowich N, Martsinkevich O, Millen L, Yuan YR, Dai PL, MacVey K, Thomas PJ, Hunt JF. Karpowich N, et al. Structure. 2001 Jul 3;9(7):571-86. doi: 10.1016/s0969-2126(01)00617-7. Structure. 2001. PMID: 11470432 - Nucleotide-dependent allostery within the ABC transporter ATP-binding cassette: a computational study of the MJ0796 dimer.
Jones PM, George AM. Jones PM, et al. J Biol Chem. 2007 Aug 3;282(31):22793-803. doi: 10.1074/jbc.M700809200. Epub 2007 May 7. J Biol Chem. 2007. PMID: 17485460 - Nucleotide binding domain interactions during the mechanochemical reaction cycle of ATP-binding cassette transporters.
Moody JE, Thomas PJ. Moody JE, et al. J Bioenerg Biomembr. 2005 Dec;37(6):475-9. doi: 10.1007/s10863-005-9494-8. J Bioenerg Biomembr. 2005. PMID: 16691486 Review. - A reciprocating twin-channel model for ABC transporters.
Jones PM, George AM. Jones PM, et al. Q Rev Biophys. 2014 Aug;47(3):189-220. doi: 10.1017/S0033583514000031. Epub 2014 Apr 30. Q Rev Biophys. 2014. PMID: 24786414 Review.
Cited by
- Converting nonhydrolyzable nucleotides to strong cystic fibrosis transmembrane conductance regulator (CFTR) agonists by gain of function (GOF) mutations.
Okeyo G, Wang W, Wei S, Kirk KL. Okeyo G, et al. J Biol Chem. 2013 Jun 14;288(24):17122-33. doi: 10.1074/jbc.M112.442582. Epub 2013 Apr 25. J Biol Chem. 2013. PMID: 23620589 Free PMC article. - Dissociation of ATP-binding cassette nucleotide-binding domain dimers into monomers during the hydrolysis cycle.
Zoghbi ME, Krishnan S, Altenberg GA. Zoghbi ME, et al. J Biol Chem. 2012 Apr 27;287(18):14994-5000. doi: 10.1074/jbc.M112.340281. Epub 2012 Mar 8. J Biol Chem. 2012. PMID: 22403405 Free PMC article. - ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer.
Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. Smith PC, et al. Mol Cell. 2002 Jul;10(1):139-49. doi: 10.1016/s1097-2765(02)00576-2. Mol Cell. 2002. PMID: 12150914 Free PMC article. - Building an understanding of cystic fibrosis on the foundation of ABC transporter structures.
Mendoza JL, Thomas PJ. Mendoza JL, et al. J Bioenerg Biomembr. 2007 Dec;39(5-6):499-505. doi: 10.1007/s10863-007-9117-7. J Bioenerg Biomembr. 2007. PMID: 18080175
References
- Young J, Holland IB. Biochim Biophys Acta. 1999;1461:177–200. - PubMed
- Higgins CF. Annu Rev Cell Biol. 1992;8:67–113. - PubMed
- Dean M, Hamon Y, Chimini G. J Lipid Res. 2001;42:1007–1017. - PubMed
- Thomas PJ, Hunt JF. Nat Struct Biol. 2001;8:920–923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources