Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus - PubMed (original) (raw)
Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus
Stephanie J Child et al. J Virol. 2002 May.
Abstract
The cellular response to viral infection often includes activation of pathways that shut off protein synthesis and thereby inhibit viral replication. In order to enable efficient replication, many viruses carry genes such as the E3L gene of vaccinia virus that counteract these host antiviral pathways. Vaccinia virus from which the E3L gene has been deleted (VVDeltaE3L) is highly sensitive to interferon and exhibits a restricted host range, replicating very inefficiently in many cell types, including human fibroblast and U373MG cells. To determine whether human cytomegalovirus (CMV) has a mechanism for preventing translational shutoff, we evaluated the ability of CMV to complement the deficiencies in replication and protein synthesis associated with VVDeltaE3L. CMV, but not UV-inactivated CMV, rescued VVDeltaE3L late gene expression and replication. Thus, complementation of the VVDeltaE3L defect appears to depend on de novo CMV gene expression and is not likely a result of CMV binding to the cell receptor or of a virion structural protein. CMV rescued VVDeltaE3L late gene expression even in the presence of ganciclovir, indicating that CMV late gene expression is not required for complementation of VVDeltaE3L. The striking decrease in overall translation after infection with VVDeltaE3L was prevented by prior infection with CMV. Finally, CMV blocked both the induction of eukaryotic initiation factor 2alpha (eIF2alpha) phosphorylation and activation of RNase L by VVDeltaE3L. These results suggest that CMV has one or more immediate-early or early genes that ensure maintenance of a high protein synthetic capacity during infection by preventing activation of the PKR/eIF2alpha phosphorylation and 2-5A oligoadenylate synthetase/RNase L pathways.
Figures
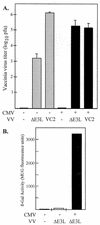
FIG. 1.
Rescue of VVΔE3L replication and late gene expression by CMV. (A) At 24 h after triplicate wells of HF had been mock infected (−, shaded bars) or infected with CMV (+, black bars), they were mock infected (−) or infected with the indicated VV. VV titers (+ standard deviation) in freeze-thaw lysates prepared 24 h later were determined by plaque assays in BHK cells. (B) At 24 h after HF had been mock infected (−) or infected with CMV (+), they were mock-infected (−) or infected with VVΔE3L. After 24 h, the β-Gal activities were measured as described in Materials and Methods.
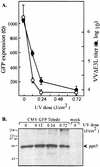
FIG. 2.
UV inactivation of CMV prevents rescue of VVΔE3L replication. HF were mock infected or infected with CMV that had been exposed to various doses of UV irradiation in quadruplicate as described in Materials and Methods. (A) After 24 h, GFP levels were measured by using a fluorescence plate reader, and then the cells were infected with VVΔE3L. At 24 h post-VVΔE3L infection, VVΔE3L titers were measured (•). GFP levels (○) represent the mean (± the standard deviation [SD]) level of expression in triplicate wells minus the background fluorescence present in mock-infected cells. VVΔE3L titers represent the means (±SD) of VVΔE3L harvested from the same triplicate wells as were used to measure GFP expression. The x axis is set at 3.8 × 103 PFU, representing the titer of VVΔE3L produced in the absence of CMV infection. (B) At 2 h post-CMV infection, samples from the same experiment were harvested and analyzed by immunoblot assay by using antibody to the CMV tegument protein pp65. Molecular size markers are indicated on the left in kilodaltons.
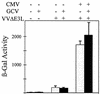
FIG. 3.
CMV rescues VVΔE3L late gene expression even in the presence of ganciclovir. Triplicate wells of HF were mock infected (−) or infected with CMV (+). At 1 hpi, cells were fed with medium containing (+, black bars) or lacking (−, shaded bars) 30 μM ganciclovir. At 24 hpi, the cells were mock infected (−) or infected with VVΔE3L (+), and β-Gal activity (+SD) was measured 24 h later.
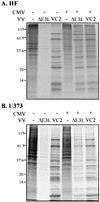
FIG. 4.
Effects of CMV on protein synthesis in VV-infected cells. HF (A) and U373MG (B) cells were mock infected (−) or infected with CMV (+), and after 24 h were mock infected (−) or infected with the indicated VV. At 24 h post-VV infection, cells were labeled with [35S]methionine for 1 h, and then 20 μg of protein in cell extracts was analyzed by SDS-PAGE and autoradiography. Molecular size markers are indicated on the left in kilodaltons.
FIG. 5.
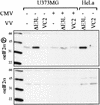
eIF2α phosphorylation after CMV and VV infection. U373MG cells were mock infected (−) or infected with CMV (+). After 24 h, the cells were mock infected (−) or infected with the indicate VV, and then lysates, prepared 24 h later, along with control extracts from VV-infected HeLa cells, were separated by SDS-PAGE and analyzed by immunoblot assay by using antibody specific for phosphorylated eIF2α (top) or total eIF2α (bottom). Molecular size markers are shown in kilodaltons to the left of each panel.
FIG. 6.
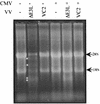
RNase L activity in CMV- and VV-infected cells. HF were mock infected (−) or infected with CMV (+). After 24 h, the cells were mock infected (−) or infected with the indicated VV. At 24 h after VV infection, RNA was harvested and ∼6 μg was visualized by formaldehyde agarose electrophoresis and SyBr green staining. RNase L cleavage products are indicated by asterisks.
Similar articles
- Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1.
Child SJ, Hakki M, De Niro KL, Geballe AP. Child SJ, et al. J Virol. 2004 Jan;78(1):197-205. doi: 10.1128/jvi.78.1.197-205.2004. J Virol. 2004. PMID: 14671101 Free PMC article. - Double-stranded RNA-binding protein E3 controls translation of viral intermediate RNA, marking an essential step in the life cycle of modified vaccinia virus Ankara.
Ludwig H, Suezer Y, Waibler Z, Kalinke U, Schnierle BS, Sutter G. Ludwig H, et al. J Gen Virol. 2006 May;87(Pt 5):1145-1155. doi: 10.1099/vir.0.81623-0. J Gen Virol. 2006. PMID: 16603515 - Double-stranded RNA binding by human cytomegalovirus pTRS1.
Hakki M, Geballe AP. Hakki M, et al. J Virol. 2005 Jun;79(12):7311-8. doi: 10.1128/JVI.79.12.7311-7318.2005. J Virol. 2005. PMID: 15919885 Free PMC article. - Translational control in virus-infected cells: models for cellular stress responses.
Clemens MJ. Clemens MJ. Semin Cell Dev Biol. 2005 Feb;16(1):13-20. doi: 10.1016/j.semcdb.2004.11.011. Epub 2004 Dec 30. Semin Cell Dev Biol. 2005. PMID: 15659335 Review. - Viral proteins targeting host protein kinase R to evade an innate immune response: a mini review.
Dzananovic E, McKenna SA, Patel TR. Dzananovic E, et al. Biotechnol Genet Eng Rev. 2018 Apr;34(1):33-59. doi: 10.1080/02648725.2018.1467151. Epub 2018 May 2. Biotechnol Genet Eng Rev. 2018. PMID: 29716441 Review.
Cited by
- Identification of a lytic-cycle Epstein-Barr virus gene product that can regulate PKR activation.
Poppers J, Mulvey M, Perez C, Khoo D, Mohr I. Poppers J, et al. J Virol. 2003 Jan;77(1):228-36. doi: 10.1128/jvi.77.1.228-236.2003. J Virol. 2003. PMID: 12477828 Free PMC article. - Murine cytomegalovirus m142 and m143 are both required to block protein kinase R-mediated shutdown of protein synthesis.
Valchanova RS, Picard-Maureau M, Budt M, Brune W. Valchanova RS, et al. J Virol. 2006 Oct;80(20):10181-90. doi: 10.1128/JVI.00908-06. J Virol. 2006. PMID: 17005695 Free PMC article. - Antagonism of Protein Kinase R by Large DNA Viruses.
Olson AT, Child SJ, Geballe AP. Olson AT, et al. Pathogens. 2022 Jul 12;11(7):790. doi: 10.3390/pathogens11070790. Pathogens. 2022. PMID: 35890034 Free PMC article. Review. - The Cellular Proteins Grb2 and DDX3 Are Increased upon Human Cytomegalovirus Infection and Act in a Proviral Fashion.
Cavignac Y, Lieber D, Laib Sampaio K, Madlung J, Lamkemeyer T, Jahn G, Nordheim A, Sinzger C. Cavignac Y, et al. PLoS One. 2015 Jun 29;10(6):e0131614. doi: 10.1371/journal.pone.0131614. eCollection 2015. PLoS One. 2015. PMID: 26121620 Free PMC article. - Who's Driving? Human Cytomegalovirus, Interferon, and NFκB Signaling.
Goodwin CM, Ciesla JH, Munger J. Goodwin CM, et al. Viruses. 2018 Aug 21;10(9):447. doi: 10.3390/v10090447. Viruses. 2018. PMID: 30134546 Free PMC article. Review.
References
- Beattie, E., E. B. Kauffman, H. Martinez, M. E. Perkus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 12:89-94. - PubMed
- Beattie, E., E. Paoletti, and J. Tartaglia. 1995. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L− and E3L− mutant viruses. Virology 210:254-263. - PubMed
- Biegalke, B. J., and A. P. Geballe. 1990. Translational inhibition by cytomegalovirus transcript leaders. Virology 177:657-667. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources