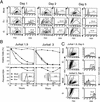
Cytopathic killing of peripheral blood CD4(+) T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env - PubMed (original) (raw)
. 2002 May;76(10):5082-93.
doi: 10.1128/jvi.76.10.5082-5093.2002.
Sara B Angleman, Viengngeun Bounkeua, Joseph Dimas, Melody G Duvall, Moses B Graubard, Felicita Hornung, Marianne C Selkirk, Christina K Speirs, Carol Trageser, Jan O Orenstein, Diane L Bolton
Affiliations
- PMID: 11967324
- PMCID: PMC136142
- DOI: 10.1128/jvi.76.10.5082-5093.2002
Cytopathic killing of peripheral blood CD4(+) T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env
Michael J Lenardo et al. J Virol. 2002 May.
Abstract
An important unresolved issue of AIDS pathogenesis is the mechanism of human immunodeficiency virus (HIV)-induced CD4(+) T-lymphocyte destruction. We show here that HIV type 1 (HIV-1) exerts a profound cytopathic effect upon peripheral blood CD4(+) T lymphocytes that resembles necrosis rather than apoptosis. Necrotic cytopathology was found with both laboratory-adapted strains and primary isolates of HIV-1. We carefully investigated the role of env, which has been previously implicated in HIV cytopathicity. HIV-1 stocks with equivalent infectivity were prepared from constructs with either an intact or mutated env coding region and pseudotyped with the glycoprotein of vesicular stomatitis virus (VSV-G) so that the HIV envelope was not rate-limiting for infection. Infected Jurkat T cells died whether or not env was intact; however, the expression of env accelerated death significantly. The accelerated death was blocked by protease inhibitors, indicating that it was due to reinfection by newly produced virus in env(+) cultures. Accordingly, we found no disparity in kinetics in CD4(lo) Jurkat cells. In highly infected peripheral blood T cells, profound necrosis occurred equivalently with both env(+) and env(-) stocks of HIV-1. We also found that HIV-1 cytopathicity was undiminished by the absence of nef. However, viral stocks made by complementation or packaging of HIV-1 genomes with the natural protein-coding sequences replaced by the green fluorescent protein were highly infectious but not cytopathic. Thus, env can accelerate cell death chiefly as an entry function, but one or more viral functions other than env or nef is essential for necrosis of CD4(+) T cells induced by HIV-1.
Figures
FIG. 1.
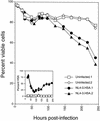
HIV-1 causes a profound cytopathic effect on cultured CD4+ T lymphocytes from peripheral blood. Purified CD4+ T lymphocytes were activated with concanavalin A and IL-2 and then infected with the NL4-3HSA strain of HIV-1. Duplicate uninfected or infected cultures were analyzed by flow cytometry for the fraction of viable cells and the expression of HSA as indicated (inset). Viable cells were determined by forward scatter versus side scatter. Infected cells are detected from cells in the viable gate. Note that early HSA is “donated” to the target cells by the virions that have acquired this membrane protein from the producer cells prior to 50 h in this experiment, whereas later HSA is due to provirus expression (11).
FIG. 2.
HIV-associated cell death does not correlate with externalization of phosphatidylserine or exposure of the mitochondrial antigen recognized by the monoclonal antibody APO2.7. Purified CD4+ T cells were infected with HIV-1 (NL4-3HSA) for 8 days or mock infected. On the ninth day, both uninfected and infected cultures were treated with staurosporine (1 μg/ml) for 7 h. The percent of viable cells was determined by FSC-SSC plots; the fraction of infected cells was measured by surface staining for HSA and, more sensitively, by internal staining for the p24 antigen. Note the lower fraction of HSA exhibited on the surface of the staurosporine-treated cells, indicating augmentation of cell death. Each panel represents 10,000 events in the live FSC-SSC gate.
FIG. 3.
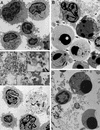
Electron micrographs of HIV-1-infected CD4+ T lymphocytes compared with staurosporine-induced apoptosis. CD4+ T lymphocytes derived from peripheral blood were activated, either infected with NL4-3HSA _env_− virus for 9 days or mock infected, and on the tenth day treated with staurosporine (1 μg/ml) for 4 h. Cells were then embedded in epoxy resin and examined by TEM. Samples include mock-infected cells (×4,900) (A), mock-infected cells with staurosporine added (×5,400) (B), infected cells (×4,900 [lower half], ×17,000 [upper half]) (C), and infected cells with staurosporine added (×7,200) (D). In panel B, the arrows indicate cells with compacted chromatin, and the arrowheads indicate apoptotic bodies. In panel C, the arrowhead indicates budding virions. The inset in panel C represents a 2.2-fold magnification of the region indicated by the arrow and illustrates the finding of mature retroviral particles within the debris of a necrotic cell.
FIG. 4.
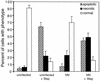
Quantitation of the level of apoptosis and necrosis in CD4+ T lymphocytes derived from peripheral blood that were either infected with NL4-3HSA for 10 days or mock infected and then treated with staurosporine (Stsp; 1 μg/ml) for 7 h. The samples were embedded in epoxy resin, sectioned, and stained with blue stain. The fractions of cells with apoptotic, necrotic, and normal appearances in the light microscope were quantified by an examiner who was blinded to the origin of the samples. The numbers in each cell sample total 100% except for slight rounding errors. Approximately 200 to 500 events were tabulated for each sample.
FIG. 5.
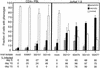
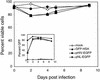
Analysis of the death effect on peripheral blood CD4+ T lymphocytes and Jurkat 1.9 T cells infected with primary isolates of HIV-1 in vitro. The samples were prepared and analyzed as in Fig. 4. Cultures 1 to 4 represent CD4+ T cells; cultures 5 to 9 represent Jurkat cells. The isolates used in each infection were as follows: cultures 1 and 5, mock-infected control; culture 2, ASM 57 virus from a long-term nonprogressor; cultures 3 and 6, dualtropic virus 302151; cultures 4 and 8, dualtropic virus 302143; culture 7, dualtropic virus 302076; and culture 9, dualtropic virus 302077. Note that the greater infectivity of Jurkat cells compared to CD4+ T cells was evident in cultures 3 and 6 as well as cultures 4 and 8, since each pair of cultures used a common primary isolate. At the bottom is provided the fraction of cells expressing p24 measured by intracytoplasmic staining and flow cytometry for each of the indicated time points. A portion of the culture was harvested for microscopy on day 11. On average, 100 to 150 cells for each culture were evaluated for morphological features of apoptosis or necrosis from photographs of stained microscope slides by a blinded observer.
FIG. 6.
NL4-3 strains lacking the envelope gene retain their cytopathic effect. (A) Dot plot and histograms exhibiting the fraction of viable cells by FSC-SSC profiles (left panels) and the fraction of infected Jurkat 1.9 cells by HSA at days 1, 2, and 5 contained within the live gate for cultures mock infected, infected with NL4-3HSA env + virus (E+), or infected with NL4-3HSA env − virus (E−) at an MOI of 10 (right panels). The numbers indicate the percentage of cells within the gates shown. (B) Quantitation of the fraction of viable cells (top panels) and the fraction of infected cells indicated by HSA (bottom panels) as a function of time for cultures of mock infected (⧫), NL4-3HSA env + virus infected (▴), or NL4-3HSA env − virus infected (▪) in independent experiments with either Jurkat 1.9 or Jurkat 3 cells as indicated. Insets show the level of CD4 on the Jurkat sublines used. (C) Dot plot and histograms of mock and E− infections exhibiting a gate of nonviable cells by FSC-SSC profiles (left panels) that were then analyzed for the percentage of infected Jurkat 1.9 cells or Jurkat 3 cells by HSA at day 5. The numbers indicate the fraction of the cells within the gates shown. The results are representative of 20 experiments.
FIG. 7.
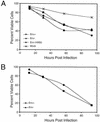
Accelerated death rate of HIV-1 NL4-3HSA env + virus-infected cells is due primarily to reinfection and is abrogated by the protease inhibitor IND. Jurkat 1.9 T cells were infected at an MOI of 1 by either NL4-3HSA env + virus or the NL4-3HSA env − virus, and 10 μM IND was added to the samples as indicated. IND was replenished daily. “Mock” indicates that no virus was added. Quantitation of the fraction of viable cells and infected cells is plotted as a function of time as in Fig. 6. Results are representative of five experiments.
FIG. 8.
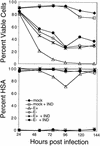
PBLs infected with either HIV-1 NL4-3HSA env + or env− strains show comparable cytopathic effects. (A) Viable cells quantitated by FSC-SSC as in Fig. 6. Samples were infected with NL4-3HSA env+ HIV-1, NL4-3HSA _env_− HIV-1, or NL4-3HSA env − HIV-1 pseudotyped with the HXB2 HIV-1 envelope or were mock infected as indicated; all HIV-1 NL4-3HSA strains used in these experiments were also pseudotyped with VSV-G protein. (B) Percent viability of highly infected PBLs as determined by high HSA expression (fluorescence intensity above the second decade). Samples were infected with HIV-1 NL4-3HSA env + virus and HIV-1 NL4-3HSA env − virus pseudotyped with VSV-G as indicated.
FIG. 9.
NL4-3 derivatives devoid of natural viral protein-coding sequences are not cytopathic. Three NL4-3 HIV-1 derivatives—HIV-GFP-HSA, pHIV-EGFP, and pNL-EGFP—that are unable to express any of the viral proteins (except for pHIV-EGFP, which expresses Tat and Rev) were used to infect Jurkat 1.9 T cells and then monitored for 9 days. The fraction of viable cells postinfection was assessed by flow cytometry as in Fig. 6, and GFP expression was examined in the viable cell population (inset). A total of 10,000 events were collected for each datum point.
Similar articles
- Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection.
Wildum S, Schindler M, Münch J, Kirchhoff F. Wildum S, et al. J Virol. 2006 Aug;80(16):8047-59. doi: 10.1128/JVI.00252-06. J Virol. 2006. PMID: 16873261 Free PMC article. - Reverted HIV-1 Mutants in CD4+ T-Cells Reveal Critical Residues in the Polar Region of Viral Envelope Glycoprotein.
Lu W, Li TW, Phillips S, Wu L. Lu W, et al. Microbiol Spectr. 2021 Dec 22;9(3):e0165321. doi: 10.1128/spectrum.01653-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34935422 Free PMC article. - Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence.
Mahlknecht U, Deng C, Lu MC, Greenough TC, Sullivan JL, O'Brien WA, Herbein G. Mahlknecht U, et al. J Immunol. 2000 Dec 1;165(11):6437-46. doi: 10.4049/jimmunol.165.11.6437. J Immunol. 2000. PMID: 11086083 - Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells?
Azad AA. Azad AA. Biochem Biophys Res Commun. 2000 Jan 27;267(3):677-85. doi: 10.1006/bbrc.1999.1708. Biochem Biophys Res Commun. 2000. PMID: 10673351 Review. - The plasma membrane as a combat zone in the HIV battlefield.
Doms RW, Trono D. Doms RW, et al. Genes Dev. 2000 Nov 1;14(21):2677-88. doi: 10.1101/gad.833300. Genes Dev. 2000. PMID: 11069884 Review. No abstract available.
Cited by
- The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest.
Sakai K, Dimas J, Lenardo MJ. Sakai K, et al. Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3369-74. doi: 10.1073/pnas.0509417103. Epub 2006 Feb 21. Proc Natl Acad Sci U S A. 2006. PMID: 16492778 Free PMC article. - Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions.
Holm GH, Gabuzda D. Holm GH, et al. J Virol. 2005 May;79(10):6299-311. doi: 10.1128/JVI.79.10.6299-6311.2005. J Virol. 2005. PMID: 15858014 Free PMC article. - Preferential cytolysis of peripheral memory CD4+ T cells by in vitro X4-tropic human immunodeficiency virus type 1 infection before the completion of reverse transcription.
Zhou Y, Shen L, Yang HC, Siliciano RF. Zhou Y, et al. J Virol. 2008 Sep;82(18):9154-63. doi: 10.1128/JVI.00773-08. Epub 2008 Jul 2. J Virol. 2008. PMID: 18596085 Free PMC article. - The vpu protein of human immunodeficiency virus type 1 plays a protective role against virus-induced apoptosis in primary CD4(+) T lymphocytes.
Komoto S, Tsuji S, Ibrahim MS, Li YG, Warachit J, Taniguchi K, Ikuta K. Komoto S, et al. J Virol. 2003 Oct;77(19):10304-13. doi: 10.1128/jvi.77.19.10304-10313.2003. J Virol. 2003. PMID: 12970415 Free PMC article. - The mechanism of necroptosis in normal and cancer cells.
Fulda S. Fulda S. Cancer Biol Ther. 2013 Nov;14(11):999-1004. doi: 10.4161/cbt.26428. Epub 2013 Sep 12. Cancer Biol Ther. 2013. PMID: 24025353 Free PMC article. Review.
References
- Accornero, P., M. Radrizzani, D. Delia, F. Gerosa, R. Kurrle, and M. P. Colombo. 1997. Differential susceptibility to HIV-GP120-sensitized apoptosis in CD4+ T-cell clones with different T-helper phenotypes: role of CD95/CD95L interactions. Blood 89:558-569. - PubMed
- Badley, A. D., D. Dockrell, and C. V. Paya. 1997. Apoptosis in AIDS. Adv. Pharmacol. 41:271-294. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials