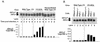Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus - PubMed (original) (raw)
Comparative Study
Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus
Ying Xiang et al. J Virol. 2002 May.
Abstract
The vaccinia virus E3L gene encodes two double-stranded RNA binding proteins that promote viral growth and pathogenesis through suppression of innate immunity. To explore how E3L enables vaccinia virus to evade the interferon system, cells and mice deficient in the principal interferon-regulated antiviral enzymes, PKR and RNase L, were infected with wild-type vaccinia virus and strains of vaccinia virus from which E3L had been deleted (E3L-deleted strains). While wild-type virus was unaffected by RNase L and PKR, virus lacking E3L replicated only in the deficient cells. Nevertheless, E3L-deleted virus failed to replicate to high titers or to cause significant morbidity or mortality in triply deficient mice lacking RNase L, PKR, and Mx1. To investigate the underlying cause, we determined the effect of E3L on interferon regulatory factor 3 (IRF3), a transcription factor required for viral induction of subtypes of type I interferons. Results showed that IRF3 activation and interferon-beta induction occurred after infections with E3L-deleted virus but not with wild-type virus. These findings demonstrate that E3L plays an essential role in the pathogenesis of vaccinia virus by blocking the interferon system at multiple levels. Furthermore, our results indicate the existence of an interferon-mediated antipoxvirus pathway that operates independently of PKR, Mx1, or the 2-5A/RNase L system.
Figures
FIG. 1.
E3L allows VV to evade the antiviral activity of RNase L. Plaque assays (A) and one-step viral-growth curves (B) of wild-type VV and VVΔE3L (Copenhagen strain) are shown. (C) Diagram of RNase L and mutant RNase LΔEN. (D) Ponasterone induction of RNase L in comparison to β-actin as determined by probing a Western blot with antibodies. (E) Assay for RNase L and mutant RNase LΔEN levels by covalent cross-linking to a 32P-labeled 2-5A analog before and after ponasterone induction (5 μM for 24 h). (F) Assay for RNase L activity in intact cells as measured by specific cleavages in rRNAs. Cells were incubated in the absence or presence of ponasterone (5 μM) for 24 h and transfected with 2-5A [p3A(2′p5′A)3] (1 μM) for 3 h. Total RNA was isolated, electrophoresed in a formaldehyde-1.2% agarose gel, stained with ethidium bromide (lower panel), and probed with 32P-labeled 18S rRNA cDNA (upper panel). (G) RNase L−/− cells containing inducible cDNAs for RNase L and RNase LΔEN were incubated in the absence or presence of ponasterone (5 μM) for 24 h and infected at an MOI of 6 with wild-type VV or VVΔE3L (Copenhagen strain). At 24 h postinfection, viruses were harvested and virus yields were determined by plaque titration on BHK21 cells.
FIG. 2.
Impact of RNase L and PKR deficiencies on viral growth and pathogenesis. (A) One-step growth of VV and VVΔE3L (Copenhagen strain) in wild-type and RNase L−/− PKR−/− cells. Cells were infected at an MOI of 6. At different times postinfection, viruses were harvested and virus yields were determined by plaque titration on BHK21 cells. (B) VV but not VVΔE3L is lethal for mice regardless of the presence or absence of RNase L and PKR. Seven-week-old wild-type, RNase L−/−, PKR−/−, and RNase L−/− PKR−/− mice were injected i.n. with 106 PFU of wild-type VV or 5 × 106 PFU of VVΔE3L (WR strain). Survival was monitored daily, and the mouse survival was plotted.
FIG. 3.
E3L blocks phosphorylation of IRF3 during VV infections. HT1080 cells (A) or wild-type or mutant MEFs (B) were infected with VV, VVΔE3L, or reconstituted wild-type VV (WR strain; MOI of 15) as indicated. IRF3 phosphorylation was monitored in Western blots probed with polyclonal antibody against IRF3 (upper panels). The ratios of phosphorylated to total IRF3 was plotted as a function of time postinfection (lower panels).
FIG. 4.
Cytoplasmic to nuclear translocalization of IRF-3 in HT1080 cells after virus infection occurs only in the absence of E3L. The subcellular localization of IRF3 is shown in mock-infected cells (A) and in cells infected with wild-type VV (B and E), VVΔE3L (C and E), or reconstituted wild-type VV (WR strain) (F and G). Infections were for 6 h (B, D, and F) or 8 h (C, E, and G). Fixed cells were stained with polyclonal anti-IRF3 antibody and then secondary Alexa 488 goat anti-rabbit antibody. Stained cells were monitored with a Leica fluorescence microscope (magnification, ×400).
FIG. 5.
Induction of IFN-β mRNA after VVΔE3L infection. HT-1080 cells were infected with either wild-type VV, VVΔE3L, or reconstituted wild-type VV (WR strain; MOI of 15) for the lengths of time indicated. The expression of IFN-β (upper panel) or GAPDH mRNA (middle panel) was detected by RT-PCR analysis. The products were analyzed on polyacrylamide gels and by autoradiography. Ethidium bromide staining was performed on the total RNA (lower panel).
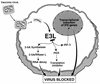
FIG. 6.
E3L of VV evades the IFN system by blocking IFN induction through IRF3 and IFN action through the 2-5A/RNase L pathway and protein kinase PKR.
Similar articles
- Roles of vaccinia virus genes E3L and K3L and host genes PKR and RNase L during intratracheal infection of C57BL/6 mice.
Rice AD, Turner PC, Embury JE, Moldawer LL, Baker HV, Moyer RW. Rice AD, et al. J Virol. 2011 Jan;85(1):550-67. doi: 10.1128/JVI.00254-10. Epub 2010 Oct 13. J Virol. 2011. PMID: 20943971 Free PMC article. - Vaccinia virus E3L protein is an inhibitor of the interferon (i.f.n.)-induced 2-5A synthetase enzyme.
Rivas C, Gil J, Mĕlková Z, Esteban M, Díaz-Guerra M. Rivas C, et al. Virology. 1998 Apr 10;243(2):406-14. doi: 10.1006/viro.1998.9072. Virology. 1998. PMID: 9568039 - Antagonizing activity of vaccinia virus E3L against human interferons in Huh7 cells.
Arsenio J, Deschambault Y, Cao J. Arsenio J, et al. Virology. 2008 Jul 20;377(1):124-32. doi: 10.1016/j.virol.2008.04.014. Epub 2008 May 27. Virology. 2008. PMID: 18502465 - The interferon system and vaccinia virus evasion mechanisms.
Perdiguero B, Esteban M. Perdiguero B, et al. J Interferon Cytokine Res. 2009 Sep;29(9):581-98. doi: 10.1089/jir.2009.0073. J Interferon Cytokine Res. 2009. PMID: 19708815 Review. - Proteins that interact with PKR.
Jagus R, Gray MM. Jagus R, et al. Biochimie. 1994;76(8):779-91. doi: 10.1016/0300-9084(94)90082-5. Biochimie. 1994. PMID: 7893827 Review.
Cited by
- The importance of IFNα2A (Roferon-A) in HSV-1 latency and T cell exhaustion in ocularly infected mice.
Wang S, Jaggi U, Katsumata M, Ghiasi H. Wang S, et al. PLoS Pathog. 2024 Oct 1;20(10):e1012612. doi: 10.1371/journal.ppat.1012612. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39352890 Free PMC article. - Vaccinia Virus: Mechanisms Supporting Immune Evasion and Successful Long-Term Protective Immunity.
Hsu J, Kim S, Anandasabapathy N. Hsu J, et al. Viruses. 2024 May 29;16(6):870. doi: 10.3390/v16060870. Viruses. 2024. PMID: 38932162 Free PMC article. Review. - Transcriptome and proteomic analysis of mpox virus F3L-expressing cells.
Wang Y, Zhang J, Li M, Jia M, Yang L, Wang T, Wang Y, Kang L, Li M, Kong L. Wang Y, et al. Front Cell Infect Microbiol. 2024 Feb 13;14:1354410. doi: 10.3389/fcimb.2024.1354410. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38415010 Free PMC article. - 2-5A-Mediated decay (2-5AMD): from antiviral defense to control of host RNA.
Prangley E, Korennykh A. Prangley E, et al. Crit Rev Biochem Mol Biol. 2022 Oct-Dec;57(5-6):477-491. doi: 10.1080/10409238.2023.2181308. Epub 2023 Mar 20. Crit Rev Biochem Mol Biol. 2022. PMID: 36939319 Free PMC article. Review. - Rotavirus NSP1 Subverts the Antiviral Oligoadenylate Synthetase-RNase L Pathway by Inducing RNase L Degradation.
Dai J, Yi G, Philip AA, Patton JT. Dai J, et al. mBio. 2022 Dec 20;13(6):e0299522. doi: 10.1128/mbio.02995-22. Epub 2022 Nov 22. mBio. 2022. PMID: 36413023 Free PMC article.
References
- Beattie, E., E. Paoletti, and J. Tartaglia. 1995. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L- and E3L-mutant viruses. Virology 210:254-263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources