TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? - PubMed (original) (raw)
TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates?
Gunnar Schröder et al. J Bacteriol. 2002 May.
Abstract
TraG-like proteins are potential NTP hydrolases (NTPases) that are essential for DNA transfer in bacterial conjugation. They are thought to mediate interactions between the DNA-processing (Dtr) and the mating pair formation (Mpf) systems. TraG-like proteins also function as essential components of type IV secretion systems of several bacterial pathogens such as Helicobacter pylori. Here we present the biochemical characterization of three members of the family of TraG-like proteins, TraG (RP4), TraD (F), and HP0524 (H. pylori). These proteins were found to have a pronounced tendency to form oligomers and were shown to bind DNA without sequence specificity. Standard NTPase assays indicated that these TraG-like proteins do not possess postulated NTP-hydrolyzing activity. Surface plasmon resonance was used to demonstrate an interaction between TraG and relaxase TraI of RP4. Topology analysis of TraG revealed that TraG is a transmembrane protein with cytosolic N and C termini and a short periplasmic domain close to the N terminus. We predict that multimeric inner membrane protein TraG forms a pore. A model suggesting that the relaxosome binds to the TraG pore via TraG-DNA and TraG-TraI interactions is presented.
Figures
FIG. 1.
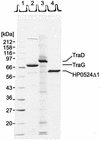
The N-terminally modified forms of TraG, TraD, and HP0524 were solubilized and purified as described in Materials and Methods. Purification steps and yields are listed in Table 4. For each protein 7 μg was loaded onto an SDS-15% polyacrylamide gel, resolved by electrophoresis, and stained with Coomassie blue R. Lane 1, marker proteins; lane 2, purified TraG (His6-TraG); lane 3, purified TraD (His6-TraD); lane 4, purified HP0524Δ1 (His6-HP0524Δ1).
FIG. 2.
TraG and TraD preparations contained ATP-hydrolyzing impurities that were removed by glycerol gradient centrifugation. Purified TraG or TraD was layered on a 15 to 35% (wt/vol) linear glycerol gradient. Centrifugation was at 270,000 × g for 38 h. Aliquots of fractions were analyzed on an SDS-15% polyacrylamide gel (top) and were assayed for ATPase activity. The graphs on the bottom show the TraG and TraD contents (in percentage of total TraG or TraD) and the ATPase activity (expressed as time-dependent release of Pi) in each fraction. The ratio of ATPase activity to protein content (Pi/TraG or TraD) is also shown. Reference proteins 1 to 3 migrated as indicated by arrows. 1, aldolase, 158 kDa, _s_20,w = 7.8; 2, BSA, 67 kDa, _s_20,w = 4.4; 3, ovalbumin, 43.5 kDa, _s_20,w = 3.6. The very broad sedimentation profiles of TraG and TraD indicate the presence of several oligomeric states of these proteins.
FIG. 3.
Trypsin sensitivity of TraG insertion mutants. Cells expressing TraG insertion mutants (TraGi31) were converted to spheroplasts and treated with (+) or without (−) trypsin prior to immunoblot analysis. Antibodies directed against the trypsin-sensitive insertion sequence were used for detection of the different mutant proteins (29).
FIG. 4.
Proposed topology of RP4 TraG. In frame TraG insertion mutants were generated for identification of periplasmic and cytoplasmic domains (Fig. 3) and for detection of nonpermissible insertion sites. Arrowheads, insertion sites. The last unaltered amino acid and its position are given. The transfer frequency of each mutant was determined in mating assays (Table 6).
FIG. 5.
TraG-like proteins of conjugal transfer systems and of other type IV secretion systems contain two conserved domains probably forming a functional NBD. Amino acid sequences of 19 different TraG-like proteins were aligned. Bars, domains 1 and 2. Conserved motifs I to V inside these domains are boxed. The origin of each protein (plasmid or strain) is indicated to the left of the sequence. Black background, amino acid positions that are conserved throughout; dark shading, identical residues present in at least 68% of the sequences; light shading, similar residues. Accession numbers are as follows: TraG (RP4), S22999; TraG (R751), S22992; VirD4 (Wolbachia sp. strain wKueYO [W.s.]), BAA97443; VirD4 (R. prowazeckii [R.p.]), H71684; VirD4 (pXF51), NP_061672; TraN (pIPO2), AJ297913 (delimited DNA sequence); MagB12 (pVT745), NP_067572; VirD4 (pTiC58/a), P18594; VirD4 (pRi1724), NP_066751; LvhD4 (L. pneumophila [L.p.]), CAB60062; TrsK (pMRC01), NP_047302; TaxB (R6K), CAA71845; TrsK (pGO1 [S.a.]), C56976; TraG (pTiC58), Q44346; HP0524 (H. pylori [H.p.]), NP_207320; TrwB (R388), S43877; TraD (pNL1), NP_049138; TraJ (pKM101), T30861; TraD (F), NP_061481.
FIG. 6.
RP4 TraG binds to both dsDNA and ssDNA with preference for ssDNA. (Left) 32P-labeled dsDNA fragments were incubated with increasing amounts of TraG and electrophoresed as described in Materials and Methods. Protein-DNA complexes were retained in the gel slots. The smallest of the dsDNA fragments (287 bp) contains the core sequence of oriT, which is the sequence specifically recognized by TraI and TraJ transfer proteins during relaxation of RP4. No binding preference for this sequence was detected for TraG. (Right) Increasing amounts of ssDNA were added as competitor to a previously incubated dsDNA-TraG mixture. dsDNA was released from TraG in the presence of small amounts of ssDNA, indicating a preference for ssDNA binding.
FIG. 7.
TraD and HP0524Δ1 bind to dsDNA. 32P-labeled dsDNA fragments were incubated with increasing amounts of TraD or HP0524Δ1 and electrophoresed as described in Materials and Methods. Protein-DNA complexes were retained in the gel slots.
FIG. 8.
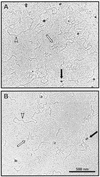
Complexes of TraG (A) and TraD (B) with ssDNA visualized by transmission electron microscopy. Equal amounts of dsDNA and ssDNA were incubated with TraG or TraD. Proteins appear as large dark spots (black arrows) and are located exclusively near ssDNA (bundles of short, thin strokes [white arrows]). dsDNA strands (white arrowheads) remain unbound in the presence of ssDNA, demonstrating the preference of TraG and TraD to bind to ssDNA.
FIG. 9.
TraG binds tightly and specifically to relaxase TraI of RP4. Real-time monitoring of complex formation between TraG and TraI is demonstrated by SPR. The TraI protein was covalently attached to the sensor chip by amide coupling. Immobilized ethanolamine (EA) and BSA (flow cells 1 and 2) served as a control for specificity. After injection, TraG protein solution flowed through flow cells 1, 2, and 3 successively (A, inset). (A) Specific binding of 30 nM TraG to immobilized TraI expressed in RU per second. The “binding curves” of nonspecific interaction with EA and BSA are displayed for comparison. At injection start (0 s) and end (120 s), the abrupt change in RU in all flow cells is due to the contribution of bulk solution on the surface layer of the sensor chip. (B) Binding of TraG to immobilized TraI at five different ligand concentrations. The association rate constant (ka) was determined by using the Langmuir binding model for 1:1 interaction between analyte and ligand.
FIG. 10.
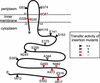
Proposed model for the RP4 relaxosome. TraG (red) is a membrane-anchored, multimeric protein probably forming a pore-like structure that could serve as a channel for translocation of the transferred ssDNA (T-DNA). The relaxase TraI (green) and the plasmid DNA both bind to this TraG pore. TraI cleaves the oriT sequence of RP4 at the nic site and is covalently attached to the 5′ end of the DNA single strand. TraJ (blue) binds to the sequence upstream of the nic site (srj) and is required for relaxase activity. TraH (yellow) is a homomultimer that stabilizes the TraI-TraJ-DNA complex, probably by bridging TraJ and TraI. TraK (orange) binds to a sequence downstream of the nic site and functions as a DNA chaperone, facilitating the formation of the TraI-DNA adduct. A helicase for displacement of the transferred strand has not been identified in the RP4 system.
Similar articles
- Interaction of Bacteroides fragilis pLV22a relaxase and transfer DNA with Escherichia coli RP4-TraG coupling protein.
Thomas J, Hecht DW. Thomas J, et al. Mol Microbiol. 2007 Nov;66(4):948-60. doi: 10.1111/j.1365-2958.2007.05967.x. Epub 2007 Oct 5. Mol Microbiol. 2007. PMID: 17919288 Free PMC article. - TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58.
Hamilton CM, Lee H, Li PL, Cook DM, Piper KR, von Bodman SB, Lanka E, Ream W, Farrand SK. Hamilton CM, et al. J Bacteriol. 2000 Mar;182(6):1541-8. doi: 10.1128/JB.182.6.1541-1548.2000. J Bacteriol. 2000. PMID: 10692358 Free PMC article. - Essential motifs of relaxase (TraI) and TraG proteins involved in conjugative transfer of plasmid RP4.
Balzer D, Pansegrau W, Lanka E. Balzer D, et al. J Bacteriol. 1994 Jul;176(14):4285-95. doi: 10.1128/jb.176.14.4285-4295.1994. J Bacteriol. 1994. PMID: 8021214 Free PMC article. - The mating pair formation system of conjugative plasmids-A versatile secretion machinery for transfer of proteins and DNA.
Schröder G, Lanka E. Schröder G, et al. Plasmid. 2005 Jul;54(1):1-25. doi: 10.1016/j.plasmid.2005.02.001. Plasmid. 2005. PMID: 15907535 Review. - Relaxosome function and conjugation regulation in F-like plasmids - a structural biology perspective.
Wong JJ, Lu J, Glover JN. Wong JJ, et al. Mol Microbiol. 2012 Aug;85(4):602-17. doi: 10.1111/j.1365-2958.2012.08131.x. Epub 2012 Jul 13. Mol Microbiol. 2012. PMID: 22788760 Review.
Cited by
- CopG1, a Novel Transcriptional Regulator Affecting Symbiosis in Bradyrhizobium sp. SUTN9-2.
Wangthaisong P, Piromyou P, Songwattana P, Phimphong T, Songsaeng A, Pruksametanan N, Boonchuen P, Wongdee J, Teamtaisong K, Boonkerd N, Sato S, Tittabutr P, Teaumroong N. Wangthaisong P, et al. Biology (Basel). 2024 Jun 5;13(6):415. doi: 10.3390/biology13060415. Biology (Basel). 2024. PMID: 38927295 Free PMC article. - Whole-Genome Sequencing and Phenotypic Analysis of Streptococcus equi subsp. zooepidemicus Sequence Type 147 Isolated from China.
Su Y, Zhang Z, Wang L, Zhang B, Su L. Su Y, et al. Microorganisms. 2024 Apr 19;12(4):824. doi: 10.3390/microorganisms12040824. Microorganisms. 2024. PMID: 38674768 Free PMC article. - Functional characterization of VirB/VirD4 and Icm/Dot type IV secretion systems from the plant-pathogenic bacterium Xanthomonas euvesicatoria.
Drehkopf S, Scheibner F, Büttner D. Drehkopf S, et al. Front Cell Infect Microbiol. 2023 Aug 1;13:1203159. doi: 10.3389/fcimb.2023.1203159. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37593760 Free PMC article. - The Missing Pieces: The Role of Secretion Systems in Campylobacter jejuni Virulence.
Gabbert AD, Mydosh JL, Talukdar PK, Gloss LM, McDermott JE, Cooper KK, Clair GC, Konkel ME. Gabbert AD, et al. Biomolecules. 2023 Jan 9;13(1):135. doi: 10.3390/biom13010135. Biomolecules. 2023. PMID: 36671522 Free PMC article. Review. - Unique TLR9 Activation by Helicobacter pylori Depends on the cag T4SS, But Not on VirD2 Relaxases or VirD4 Coupling Proteins.
Tegtmeyer N, Linz B, Yamaoka Y, Backert S. Tegtmeyer N, et al. Curr Microbiol. 2022 Mar 3;79(4):121. doi: 10.1007/s00284-022-02813-9. Curr Microbiol. 2022. PMID: 35239059 Free PMC article.
References
- Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. - PubMed
- Backert, S., E. Nickisch-Rosenegk, and T. F. Meyer. 1998. Potential role of two Helicobacter pylori relaxases in DNA transfer? Mol. Microbiol. 30:673-674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous