Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II - PubMed (original) (raw)
Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II
Chia-Jen Siao et al. J Neurosci. 2002.
Abstract
Microglia are the immunocompetent cells of the CNS, and their activation is thought to play an important neurotoxic role in many diseases modeled by glutamate-induced excitotoxicity. One molecule whose expression is upregulated after excitotoxic injury is tissue plasminogen activator (tPA), a serine protease with dual roles in the CNS. The catalytic activity of tPA, which converts plasminogen into plasmin, leads to neuronal death during excitotoxicity. Via a nonproteolytic mechanism, tPA also mediates microglial activation. We show here in culture studies that stimulated wild-type neurons and microglia can release the tPA that elicits the activation, and that tPA acts in combination with other factors. We also show that the finger domain of tPA is necessary to trigger the activation and identify annexin II as its probable binding partner-receptor. Together, these findings suggest that tPA released by either neurons or microglia can act as a neural cytokine, signaling through annexin II to activate microglia in settings of disease and injury. Developing methods to inhibit the interaction of tPA with annexin II would offer a new and selective approach to interfere with microglial activation for therapeutic purposes.
Figures
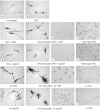
Fig. 1.
tPA−/− microglia in culture respond to signals released from injured neurons and microglia by becoming activated and changing morphology. Cells were treated as described in Results, and the expression of F4/80 was examined.a, Control, untreated tPA−/−microglia (400× magnification). b, 100 ng/ml LPS (400× magnification). c, CM prepared from quiescent tPA−/− neurons (400× magnification).d, CM prepared from glutamate (Glu)-stimulated tPA−/− neurons (400× magnification). e, Same as d(100× magnification). f, CM prepared from glutamate-stimulated tPA−/− neurons additionally containing tPA at 500 ng/ml (100× magnification). g, CM prepared from quiescent tPA−/− microglia (400× magnification). h, CM prepared from LPS-stimulated tPA−/− microglia. The LPS was partially removed using a polymyxin B column before addition of the CM to the responding tPA−/− microglia (400× magnification).i, Same as h (100× magnification). j, CM prepared from LPS-stimulated tPA−/− microglia additionally containing tPA at 500 ng/ml. The LPS was partially removed using a polymyxin B column before addition of the CM to the responding tPA−/−microglia (100× magnification). k, CM prepared from quiescent wild-type neurons (400× magnification). l, CM prepared from glutamate-stimulated wild-type neurons (400× magnification). m, Same as l (100× magnification). n, CM prepared from α-tPA antibody of glutamate-stimulated wild-type neurons (100× magnification).o, CM prepared from quiescent wild-type microglia (400× magnification). p, CM prepared from LPS-stimulated wild-type microglia. The LPS was partially removed using a polymyxin B column before addition of the CM to the responding tPA−/− microglia (400× magnification).q, Same as p (100× magnification).r, CM prepared from α-tPA antibody of LPS-stimulated wild-type microglia. The LPS was partially removed using a polymyxin B column before addition of the CM to the responding tPA−/− microglia (100× magnification).Arrowheads, Membrane ruffles or pseudopods.Arrows, Phagocytic vacuoles.
Fig. 2.
tPA and LPS are required to activate tPA−/− microglia. Microglia were cultured as indicated and then fixed and immunostained for F4/80 expression.a, Not treated; b, 5 ng/ml tPA;c, 1 μg/ml tPA; d, 100 ng/ml LPS;e, 5 ng/ml tPA, LPS; f, 500 ng/ml tPA, LPS. All panels are shown at 100× magnification.
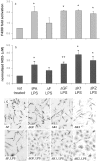
Fig. 3.
The finger domain of tPA mediates microglial activation. tPA−/− microglia were cultured with LPS in combination with full-length tPA (tPA LPS), tPA lacking the finger domain (ΔF LPS) or the growth factor domain (ΔGF LPS), or either of the kringle domains (ΔK1 LPS and ΔK2 LPS).a, Quantification of F4/80 expression by Western blot.b, Detection of NO release by its product, nitrite (NO2−). *p < 0.01; **p < 0.05. Data expressed are averages ± SEM of triplicate experiments; all analyses were conducted using Student's t test relative to the control cells that did not receive additions to the culture. c, tPA−/− microglia were cultured on coverslips under the indicated conditions and then immunostained for F4/80 expression; panels are shown at 200× magnification.
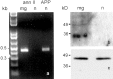
Fig. 4.
Primary microglia, but not neurons, express annexin II, a potential tPA receptor. a, RT-PCR results from microglia (mg) or neurons (n), amplified using either annexin II (ann II) or APP primers. The quality of the neuronal cDNA was established using the APP as a positive control (lane 4). b, Western blot confirming that primary microglia but not neurons express annexin II. The cell lysates used for the Western blot were also probed with the neuronal marker microtubule-associated protein 2 and the microglial marker F4/80 to confirm that the respective cell types had been highly enriched as intended and indicated (data not shown). c, An anti-actin antibody was used to ascertain equal protein loading for the lysates analyzed on the Western blot.
Fig. 5.
tPA-mediated microglial activation is blocked by α-annexin II antibodies. a, Measurement of F4/80 expression by Western blot. tPA−/− microglia were cultured with medium only [not treated (n.t.)]; LPS and 500 ng/ml tPA (tPA, LPS); LPS and 500 ng/ml ΔF mutant (ΔF, LPS); 1 μg/ml anti-annexin II (α-annII); or LPS, 1 μg/ml anti-annexin II, and 500 ng/ml tPA (α-annII, tPA,LPS). Data from two independent experiments were pooled and plotted as fold activation over the control cells, which received no additions. Error bars indicate range of data points. b–e, tPA−/−microglia were cultured alone or with α-annexin II or α-LRP antibodies for 1 hr, 500 ng/ml tPA was added for another hour, and then LPS was added and the cells were incubated overnight. Activation was examined using F4/80 immunocytochemistry on fixed cells as described in Materials and Methods. The panels are shown at 400× magnification.
Similar articles
- Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury.
Siao CJ, Fernandez SR, Tsirka SE. Siao CJ, et al. J Neurosci. 2003 Apr 15;23(8):3234-42. doi: 10.1523/JNEUROSCI.23-08-03234.2003. J Neurosci. 2003. PMID: 12716930 Free PMC article. - Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system.
Rogove AD, Siao C, Keyt B, Strickland S, Tsirka SE. Rogove AD, et al. J Cell Sci. 1999 Nov;112 ( Pt 22):4007-16. doi: 10.1242/jcs.112.22.4007. J Cell Sci. 1999. PMID: 10547361 - The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain.
Zhang C, An J, Strickland DK, Yepes M. Zhang C, et al. Am J Pathol. 2009 Feb;174(2):586-94. doi: 10.2353/ajpath.2009.080661. Epub 2009 Jan 15. Am J Pathol. 2009. PMID: 19147818 Free PMC article. - Clinical implications of the involvement of tPA in neuronal cell death.
Tsirka SE. Tsirka SE. J Mol Med (Berl). 1997 May;75(5):341-7. doi: 10.1007/s001090050119. J Mol Med (Berl). 1997. PMID: 9181475 Review. - Tissue plasminogen activator as a modulator of neuronal survival and function.
Tsirka SE. Tsirka SE. Biochem Soc Trans. 2002 Apr;30(2):222-5. Biochem Soc Trans. 2002. PMID: 12023855 Review.
Cited by
- Tuftsin promotes an anti-inflammatory switch and attenuates symptoms in experimental autoimmune encephalomyelitis.
Wu M, Nissen JC, Chen EI, Tsirka SE. Wu M, et al. PLoS One. 2012;7(4):e34933. doi: 10.1371/journal.pone.0034933. Epub 2012 Apr 17. PLoS One. 2012. PMID: 22529957 Free PMC article. - Inflammatory Cytokine Profile and Plasticity of Brain and Spinal Microglia in Response to ATP and Glutamate.
Jesudasan SJB, Gupta SJ, Churchward MA, Todd KG, Winship IR. Jesudasan SJB, et al. Front Cell Neurosci. 2021 Apr 6;15:634020. doi: 10.3389/fncel.2021.634020. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33889075 Free PMC article. - Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS.
Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, Fox HS. Roberts ES, et al. Am J Pathol. 2003 Jun;162(6):2041-57. doi: 10.1016/S0002-9440(10)64336-2. Am J Pathol. 2003. PMID: 12759259 Free PMC article. - Conditional deletion of neuronal cyclin-dependent kinase 5 in developing forebrain results in microglial activation and neurodegeneration.
Takahashi S, Ohshima T, Hirasawa M, Pareek TK, Bugge TH, Morozov A, Fujieda K, Brady RO, Kulkarni AB. Takahashi S, et al. Am J Pathol. 2010 Jan;176(1):320-9. doi: 10.2353/ajpath.2010.081158. Epub 2009 Nov 30. Am J Pathol. 2010. PMID: 19948833 Free PMC article. - VEGF receptor antagonist Cyclo-VEGI reduces inflammatory reactivity and vascular leakiness and is neuroprotective against acute excitotoxic striatal insult.
Ryu JK, McLarnon JG. Ryu JK, et al. J Neuroinflammation. 2008 May 20;5:18. doi: 10.1186/1742-2094-5-18. J Neuroinflammation. 2008. PMID: 18492281 Free PMC article.
References
- Akiyama H, Ikeda K, Kondo H, McGeer P, McGeer E. Immunohistochemical study of type-1 plasminogen activator inhibitor (PAI-1) in brain. Soc Neurosci Abstr. 1995;21:741.
- Benveniste E. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–173. - PubMed
- Bu G, Morton P, Schwartz A. Identification and partial characterization by chemical cross-linking of a binding protein for tissue-type plasminogen activator (t-PA) on rat hepatoma cells. J Biol Chem. 1992;267:15595–15602. - PubMed
- Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord J, Collen D, Mulligan R. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. - PubMed
- Chen Z-L, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources