Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region - PubMed (original) (raw)
Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region
Pietro Paolo Sanna et al. J Neurosci. 2002.
Erratum in
- J Neurosci 2002 Dec 1;22(23):10507
Abstract
Several signal transduction pathways have been implicated in the induction of long-term potentiation (LTP), yet the signal transduction mechanisms behind the maintenance-expression phase of LTP are still poorly understood. We investigated the role of phosphatidylinositol 3-kinase (PI3-kinase) in LTP at Schaffer collateral/commissural fiber-CA1 synapses in rat hippocampal slices using biochemical approaches and extracellular electrophysiological recordings. We observed that PI3-kinase activity was induced in the CA1 region during LTP of field EPSPs (fEPSPs) and that two structurally unrelated PI3-kinase inhibitors, LY294002 and wortmannin, abated established LTP, suggesting that PI3-kinase is involved in the maintenance-expression phase of LTP. However, LTP recovered after washout of the reversible PI3-kinase inhibitor LY294002, confirming that LTP maintenance and expression are distinct events and indicating that PI3-kinase activity is required for LTP expression rather than for its maintenance. Interestingly, preincubation with LY294002 did not prevent LTP induction. In fact, if LY294002 was withdrawn 5 min after high-frequency stimulation, an LTP of fEPSP was seen. Last, a voltage-dependent calcium channel-dependent form of LTP in the CA1 could also be reversibly abated by LY294002, raising the possibility that PI3-kinase could be required for the expression of multiple forms of synaptic potentiation.
Figures
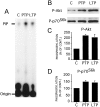
Fig. 1.
PI3-kinase and its downstream effectors Akt and p70S6K are activated in LTP. A, PI3-kinase activity was increased after LTP-inducing HFS. PI3-kinase activity was tested in CA1 protein extracts using phosphatidylinositol as a substrate in the presence of [γ-32P]ATP. Accumulation of radioactive phosphatidylinositol-3P (PIP) was increased shortly after HFS (PTP) and 30 min after HFS (LTP) when compared with untetanized control slices (C). B–D, Phosphorylation of PI3-kinase downstream effectors Akt and p70S6K was also significantly increased (p < 0.05 for both), as revealed by Western blotting with specific phosphorylation state-specific antibodies. C, D, Mean phosphorylation of Akt and p70S6K, respectively; error bars indicate SEM. *p < 0.05 indicates different from control. E, Incubation of hippocampal slices with the PI3-kinase-specific inhibitor LY294002 (100 μ
m
) prevented increased phosphorylation of Akt and p70S6K 30 min after HFS; representative blots.F, Cumulative results from these experiments. *p < 0.05 indicates different from basal; ‡p < 0.05 indicates different from potentiated slices.
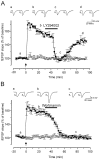
Fig. 2.
Inhibitors of PI3-kinase abate established LTP of fEPSP in the CA1 region. Synaptic potentials were simultaneously monitored in two independent pathways [white circles, stimulus 1 (S1); black squares, stimulus 2 (S2)]. The structurally unrelated inhibitors of PI3-kinase LY294002 (100 μ
m
) and wortmannin (5 μ
m
) were applied 30 min after delivery of HFS to one of the two pathways (S2).Insets are representative traces of extracellular fEPSPs recorded at the times marked by lowercase letters. Each representative trace is an average of five responses. Graphs represent the mean normalized fEPSP slopes plotted against time.Arrows indicate when tetanic stimulation to one pathway (black squares) was given at time 0. A, A transient 20 min application of LY294002 (100 μ
m
) 30 min after LTP induction abated LTP in the potentiated pathway (n = 7) (black squares), but no change was seen in the untetanized pathway (white circles). B, Similar results were obtained with wortmannin, a structurally unrelated PI3-kinase inhibitor (5 μ
m
) (n = 5). As expected, inhibition by wortmannin was irreversible.
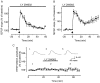
Fig. 3.
PI3-kinase is not required for LTP induction.A, Hippocampal slices continuously incubated in LY294002 (100 μ
m
) did not display LTP of fEPSP after delivery of HFS (n = 6). B, If LY294002 was withdrawn 5 min after HFS, an LTP of fEPSP was seen (n = 6). C, Pharmacologically isolated NMDA receptor-mediated EPSP amplitudes were not modified by a 20 min application of LY294002 (100 μ
m
) (102.2 ± 2.5%) as revealed by intracellular recordings from CA1 pyramidal neurons (n = 5).
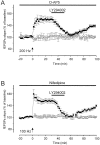
Fig. 4.
PI3-kinase is required for the expression of both NMDA- and VDCC-dependent forms of CA1 LTP.A, L-type VDCC-dependent form of LTP of fEPSP was induced at Schaffer collateral/commissural fiber–CA1 synapses in the presence of
d
-AP-5 (50 μ
m
). A 20 min application of LY294002 (100 μ
m
) 30 min after the induction of VDCC-dependent LTP abated LTP in the tetanized pathway (n = 6) (black squares), but no change was seen in the fEPSP in the untetanized pathway (white circles). B, Inhibition of PI3-kinase with LY294002 (100 μ
m
) also abated NMDA-dependent LTP induced in the presence of nifedipine (30 μ
m
).
Fig. 5.
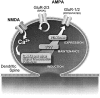
A model for the role of PI3-kinase in LTP. AMPA receptors composed of subunits 2 and 3 (GluR2/3) are believed to be responsible for basal AMPA responses. They are present at variable levels at postsynaptic sites in which they constitutively cycle between intracellular compartments and presynaptic membranes (Passafaro et al., 2001; Shi et al., 2001). Addition of GluR1 AMPA receptors in complex with GluR2 (GluR1/2) to postsynaptic membranes is believed to be the basis for LTP (Shi et al., 2001). We showed here that PI3-kinase is required for LTP but not for basal AMPA transmission. This observation is consistent with a role for PI3-kinase in the insertion of AMPA receptors during LTP but not in regulating basal AMPA receptor density. Consistent with this view, it has been shown recently that NMDA-dependent exocytosis of GluR1-containing AMPA receptor in primary hippocampal neurons is PI3-kinase dependent, unlike the exocytosis of GluR2 subunits not associated with GluR1 (Passafaro et al., 2001). Interestingly, delivery of HFS during perfusion with the reversible PI3-kinase inhibitor LY294002 did not prevent expression of LTP after washout of this inhibitor, and addition of LY294002 to established LTP caused a reversible inhibition of LTP. The former observation suggests that PI3-kinase is not responsible for the induction of LTP. The latter observation confirms that LTP maintenance and expression are distinct events (Malinow et al., 1988) and indicates that PI3-kinase activity is required for LTP expression rather than for its maintenance.
Similar articles
- Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms.
Opazo P, Watabe AM, Grant SG, O'Dell TJ. Opazo P, et al. J Neurosci. 2003 May 1;23(9):3679-88. doi: 10.1523/JNEUROSCI.23-09-03679.2003. J Neurosci. 2003. PMID: 12736339 Free PMC article. - Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons.
Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT. Man HY, et al. Neuron. 2003 May 22;38(4):611-24. doi: 10.1016/s0896-6273(03)00228-9. Neuron. 2003. PMID: 12765612 - An overview of extracellular field potentials: Different potentiation and measurable components, interpretations, and hippocampal synaptic activity models.
Radahmadi M, Halabian A, Halabian A. Radahmadi M, et al. Methods. 2025 Jul;239:50-63. doi: 10.1016/j.ymeth.2025.03.015. Epub 2025 Mar 25. Methods. 2025. PMID: 40147603 Review. - A biochemist's view of long-term potentiation.
Roberson ED, English JD, Sweatt JD. Roberson ED, et al. Learn Mem. 1996 Jul-Aug;3(1):1-24. doi: 10.1101/lm.3.1.1. Learn Mem. 1996. PMID: 10456072 Review.
Cited by
- Impairment of synaptic plasticity in the primary somatosensory cortex in a model of diabetic mice.
García-Magro N, Mesa-Lombardo A, Barros-Zulaica N, Nuñez Á. García-Magro N, et al. Front Cell Neurosci. 2024 Jul 30;18:1444395. doi: 10.3389/fncel.2024.1444395. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39139399 Free PMC article. - Phosphorylation changes of CaMKII, ERK1/2, PKB/Akt kinases and CREB activation during early long-term potentiation at Schaffer collateral-CA1 mouse hippocampal synapses.
Racaniello M, Cardinale A, Mollinari C, D'Antuono M, De Chiara G, Tancredi V, Merlo D. Racaniello M, et al. Neurochem Res. 2010 Feb;35(2):239-46. doi: 10.1007/s11064-009-0047-0. Neurochem Res. 2010. PMID: 19731018 - Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1.
Cammalleri M, Lütjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Cammalleri M, et al. Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):14368-73. doi: 10.1073/pnas.2336098100. Epub 2003 Nov 17. Proc Natl Acad Sci U S A. 2003. PMID: 14623952 Free PMC article. - Neuron-specific regulation of class I PI3K catalytic subunits and their dysfunction in brain disorders.
Gross C, Bassell GJ. Gross C, et al. Front Mol Neurosci. 2014 Feb 13;7:12. doi: 10.3389/fnmol.2014.00012. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24592210 Free PMC article. Review.
References
- Akasaki T, Koga H, Sumimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J Biol Chem. 1999;274:18055–18059. - PubMed
- Balendran A, Currie R, Armstrong CG, Avruch J, Alessi DR. Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J Biol Chem. 1999;274:37400–37406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AA006420/AA/NIAAA NIH HHS/United States
- MH64376/MH/NIMH NIH HHS/United States
- R21 MH064376/MH/NIMH NIH HHS/United States
- P50AA06420/AA/NIAAA NIH HHS/United States
- MH62140/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous