3beta -hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists - PubMed (original) (raw)
. 2002 May 1;22(9):3366-75.
doi: 10.1523/JNEUROSCI.22-09-03366.2002.
Yejun He, Lawrence N Eisenman, Christopher Fields, Chun-Min Zeng, Jose Mathews, Ann Benz, Tao Fu, Erik Zorumski, Joe Henry Steinbach, Douglas F Covey, Charles F Zorumski, Steven Mennerick
Affiliations
- PMID: 11978813
- PMCID: PMC6758359
- DOI: 10.1523/JNEUROSCI.22-09-03366.2002
3beta -hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists
Mingde Wang et al. J Neurosci. 2002.
Abstract
Endogenous neurosteroids have rapid actions on ion channels, particularly GABA(A) receptors, which are potentiated by nanomolar concentrations of 3alpha-hydroxypregnane neurosteroids. Previous evidence suggests that 3beta-hydroxypregnane steroids may competitively antagonize potentiation induced by their 3alpha diastereomers. Because of the potential importance of antagonists as experimental and clinical tools, we characterized the functional effect of 3beta-hydroxysteroids. Although 3beta-hydroxysteroids reduced the potentiation induced by 3alpha-hydroxysteroids, 3beta-hydroxysteroids acted noncompetitively with respect to potentiating steroids and inhibited the largest degrees of potentiation most effectively. Potentiation by high concentrations of barbiturates was also reduced by 3beta-hydroxysteroids. 3beta-Hydroxysteroids are also direct, noncompetitive GABA(A) receptor antagonists. 3beta-Hydroxysteroids coapplied with GABA significantly inhibited responses to > or =15 microm GABA. The profile of block was similar to that exhibited by sulfated steroids, known blockers of GABA(A) receptors. This direct, noncompetitive effect of 3beta-hydroxysteroids was sufficient to account for the apparent antagonism of potentiating steroids. Mutated receptors exhibiting decreased sensitivity to sulfated steroid block were insensitive to both the direct effects of 3beta-hydroxysteroids on GABA(A) responses and the reduction of potentiating steroid effects. At concentrations that had little effect on GABAergic synaptic currents, 3beta-hydroxysteroids and low concentrations of sulfated steroids significantly reversed the potentiation of synaptic currents induced by 3alpha-hydroxysteroids. We conclude that 3beta-hydroxypregnane steroids are not direct antagonists of potentiating steroids but rather are noncompetitive, likely state-dependent, blockers of GABA(A) receptors. Nevertheless, these steroids may be useful functional blockers of potentiating steroids when used at concentrations that do not affect baseline neurotransmission.
Figures
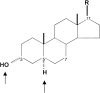
Fig. 1.
Steroid structure, with emphasis (arrows) on the chiral centers at C3 and C5. The structure shown is 3α,5α,17β. 3β-Hydroxypregnane steroids have been suggested to antagonize the potentiating actions of steroids with a 3α-hydroxy configuration. For the steroids tested in this work, the R group was CN, COCH3, or COCH2OH.
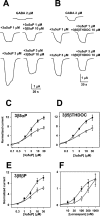
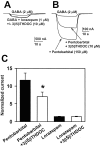
Fig. 2.
3β5αP and 3β5βTHDOC reversed the effect of high concentrations of GABA potentiating steroids. A, Sample traces showing that 10 μ
m
3β5αP inhibited potentiation by high but not low concentrations of 3α5αP.B, Similar traces for 10 μ
m
3β5βTHDOC.C–E, Summary of effect of 3β5αP (C), 3β5βTHDOC (D), and 3β5βP (E) from oocytes tested with a range of 3α5αP concentrations. C, Summary of the effect of 10 μ
m
3β5αP against increasing concentrations of 3α5αP. Normalized responses in this and subsequent figures were calculated as follows: (_I_M −_I_N)/_I_N, where _I_M is the amplitude of the measured current in a given experimental condition, and_I_N is the normalizing current. For these data and data in D and E,_I_N was the response to 2 μ
m
GABA in the absence of modulator. The solid lines_represent least-squares fits of the data to the Hill equation as follows: I = I_max×_C n/(EC50_n_+ C n), where C is the concentration of potentiator, EC50 is the concentration of potentiator that produced half-maximum potentiation, and_n is the Hill coefficient. Parameters of the fit for potentiators in the absence (filled circles) and presence (open squares) of 3β5αP are given in Table 1. D, Summary of the effect of 10 μ
m
3β5βTHDOC under similar experimental conditions to those in C. E, Concentration–response relationship for 3α5αP in the presence and absence of 3β5βP (10 μ
m
). F, Concentration–response relationship of the benzodiazepine agonist lorazepam in the presence and absence of the benzodiazepine antagonist flumazenil. The solid line through lorazepam concentration–response values (circles) is the best fit of the Hill equation, with an EC50 of 76.7 n
m
and a Hill coefficient of 1.2 (n = 7). The solid line through lorazepam–flumazenil interaction values (squares) is the best fit of the Hill equation, with an EC50 of 348.5 n
m
and a Hill coefficient of 1.1 (n = 7).
Fig. 3.
Effects of a series 3β-hydroxysteroids on 2 μ
m
GABA responses in the presence and absence of 3α5αP. A, Effects of several 3β-hydroxysteroids (10 μ
m
) against potentiation induced by 3α5αP (3 μ
m
) in the presence of 2 μ
m
GABA. For these data, the normalizing response (_I_N, representing 0 on the _y_-axis) was the current in the combined presence of 2 μ
m
GABA plus 3 μ
m
3α5αP (n = 6). B, Effects of several 3β-hydroxysteroids on responses to 2 μ
m
GABA alone. Most compounds were effectively inert at 10 μ
m
, except for 3β5βP, which slightly potentiated responses to 2 μ
m
GABA. _I_N represented the response to 2 μ
m
GABA alone (n = 5).
Fig. 4.
Effect of 3β-hydroxypregnane steroids on other 5α-reduced and 5β-reduced steroid potentiators of GABAAresponses. A, Concentration dependence of 3α5αTHDOC potentiation in the absence (filled circles) and presence (open squares) of 10 μ
m
3β5βTHDOC. GABA (2 μ
m
) was coapplied with steroids, and the response to 2 μ
m
GABA was used as the normalizing response (I_N). B,C, Similar analyses using the 5β-reduced steroids 3α5βP and 3α5βTHDOC as potentiators. In all_panels, the solid line through the concentration–response values in the absence and presence of 3β5βTHDOC is the best fit of the Hill equation. Parameters of the fits and n values are given in Table 1.
Fig. 5.
Effect of 3β-hydroxysteroids on two other classes of GABAA receptor potentiators. A, Effect of 3β5βTHDOC on potentiation by the benzodiazepine lorazepam in an oocyte. B, Effect of 3β5βTHDOC on potentiation by the barbiturate pentobarbital in another oocyte. C, Summary of the effects of 10 μ
m
3β5βTHDOC on pentobarbital (150 μ
m
; n = 13) and lorazepam (1 μ
m
; n = 5) potentiation. Note that the more robust potentiation by the barbiturate was more effectively antagonized. *p < 0.01 indicates significant inhibition.
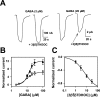
Fig. 6.
3β-Hydroxypregnane steroids block responses to high concentrations of GABA. A, Sample traces showing lack of effect of 10 μ
m
3β5βTHDOC on responses to 2 μ
m
GABA but inhibition of responses to 20 μ
m
GABA. Drugs were coapplied for 20 sec. Note the change in vertical calibration bars between the_left_ and right panels. B, GABA concentration–response curves in the absence (filled circles) and presence (open squares) of 10 μ
m
3β5βTHDOC. Each_point_ is calculated relative to the normalizing response (_I_N) activated by 2 μ
m
GABA. The solid line through GABA concentration–response values (circles) is the best fit of the Hill equation, with an EC50 of 12.6 μ
m
and a Hill coefficient of 2.6 (n = 8). The_solid line_ through GABA plus 3β5βTHDOC interaction values (squares) is the best fit of the Hill equation, with an EC50 of 9.4 μ
m
and a Hill coefficient of 2.1 (n = 8). C. The graph shows the concentration–response curve of 3β5βTHDOC against 20 μ
m
GABA. The normalizing current (_I_N) was the response to 20 μ
m
GABA. The solid lines are the fit of the Hill equation, with an IC50 of 6.8 μ
m
and a Hill coefficient of 0.8, with maximum inhibition of −1.0 (n = 6).
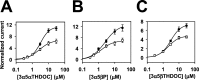
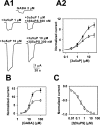
Fig. 7.
The action of 3β-hydroxysteroids is similar to that of sulfated steroid block. A, Inhibition of potentiation by a sulfated steroid. A1, Sample traces showing the very small effect of 300 n
m
3β5αPS against potentiation by 1 μ
m
3α5αP but stronger inhibition against GABA responses potentiated by 10 μ
m
3α5αP.A2, Summary of experiments like that shown in_A1_, in which multiple concentrations of potentiator were examined. GABA (2 μ
m
) was coapplied with varied concentrations of 3α5αP either without (filled circles) or with (open squares) 300 n
m
3β5αPS. Parameters of fits to the Hill equation and experimental n are given in Table 1. B, Sulfated steroid block, like 3β-hydroxysteroid block, is dependent on GABA concentration. Shown are concentration– response curves for GABA in the absence (filled circles) and presence (open squares) of 300 n
m
3β5αPS. In the absence of steroid, the EC50 for GABA was 12.5 μ
m,
with a Hill coefficient of 2.8 (_n_= 7). In the presence of steroid, the GABA EC50 was 11.4 μ
m,
with a Hill coefficient of 3.0 (_n_= 7). The normalized maximum response was decreased from 13.9 to 7.8 in the presence of steroid. C, Against a fixed GABA concentration of 20 μ
m
, 3β5αPS produced a concentration-dependent inhibition of responses. The solid line is a fit of the Hill equation, with an IC50 of 189 n
m
and a Hill coefficient of 0.7 (_n_= 6).
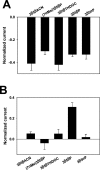
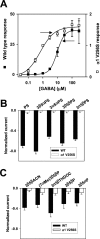
Fig. 8.
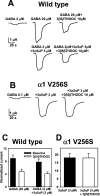
A point mutation in the α1 subunit (V256S) reduces sulfate and 3β-hydroxysteroid block of GABAAreceptors. A, GABA concentration–response curves for wild-type (filled squares; _n_= 5) and mutated (open squares; n = 6) GABAA receptors. Responses at 1 μ
m
were set to 1 for both wild-type and mutated receptors, and other responses in the same oocyte were normalized to this response. Note that the_left_ _y_-axis corresponds to the wild-type normalized responses, and the right _y_-axis corresponds to the normalized responses from mutated receptors. Absolute amplitudes of maximum responses did not notably differ between wild-type and mutated receptors, but the mutation resulted in an ∼20-fold leftward shift in the GABA EC50, from 9.6 to 0.53 μ
m
, resulting in the apparently larger normalized maximum responses for wild-type receptors. Arrows indicate concentrations of GABA used in subsequent studies of steroid block of GABAA receptors (B, C). B, Sulfated steroid effects (1 μ
m
) in wild-type (WT,filled bars; n = 5) and α1V256S mutated (open bars; n = 5) GABAA receptors. C, 3β-Hydroxysteroid effects (10 μ
m
) in wild-type (WT,filled bars; n = 6) and α1V256S (open bars; n = 9) mutated GABAA receptors.
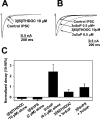
Fig. 9.
Direct GABAA receptor block explains 3β-hydroxysteroid inhibition of potentiation. A, Comparison of responses “potentiated” by increasing GABA concentration or by adding 3 μ
m
3α5αP. The_traces_ show that 20 μ
m
GABA and 3 μ
m
3α5αP cause a similar increase in current relative to 2 μ
m
GABA. 3β5βTHDOC (10 μ
m
) inhibited both increased currents to a similar degree.B, Mutated receptors are potentiated by 3α-hydroxysteroids, but steroid potentiation is not inhibited by 3β-hydroxysteroids. C, Summary of the blocking actions of 3β5βTHDOC on 20 μ
m
GABA-activated current and potentiation of 2 μ
m
GABA-activated current by 3 μ
m
3α5αP (n = 7) in oocytes expressing wild-type receptors. The bar graph represents current amplitudes normalized to the 2 μ
m
GABA response.D, The bar graph represents a summary of the lack of effect of 3β5βTHDOC on potentiation by 3α5αP in mutated receptors. Normalizing current was the response activated by 0.1 μ
m
GABA alone (n = 8).
Fig. 10.
Similar synaptic effect of 3β5βTHDOC and 3β5αPS. A, Autaptic IPSCs were elicited from solitary GABAergic hippocampal neurons in microcultures with 2 msec voltage pulse to 0 mV from a holding potential of −70 mV. The_traces_ represent responses obtained in the absence and presence of 10 μ
m
3β5βTHDOC. Note that the drug alone only slightly affected the IPSC. B, The_traces_ represent responses obtained in the absence and presence of 0.5 μ
m
3α5αP and 0.5 μ
m
3α5αP plus 10 μ
m
3β5βTHDOC. Note that 3α5αP significantly prolonged the decay time course of the IPSC. 3β5βTHDOC inhibited the prolongation induced by potentiating steroid. C, Summary of the effect of 10 μ
m
3β5βTHDOC and 2 μ
m
3β5αPS on IPSCs in the absence and presence of 0.5 μ
m
3α5αP (n = 6 neurons). For this analysis, we used 10–90% decay times, which averaged 315 ± 71 msec in the control situation. We also fit IPSC decays with multiple exponential components (Zorumski et al., 1998). Weighted time constants (∑_a_iτI, where_a_i is the fractional amplitude and τI is the time constant of each exponential component) were also used to quantify the data (Jones and Westbrook, 1997). Raw values from the weighted time constant analysis were 137 ± 30 msec (control), 119 ± 20 msec (3β5βTHDOC alone), 101 ± 18 msec (3β5αPS alone), 486 ± 63 msec (3α5αP alone), 176 ± 20 msec (3α5αP plus 3β5βTHDOC), and 266 ± 48 msec (3α5αP plus 3β5αPS).
Similar articles
- Neurosteroid modulation of recombinant rat alpha5beta2gamma2L and alpha1beta2gamma2L GABA(A) receptors in Xenopus oocyte.
Rahman M, Lindblad C, Johansson IM, Bäckström T, Wang MD. Rahman M, et al. Eur J Pharmacol. 2006 Oct 10;547(1-3):37-44. doi: 10.1016/j.ejphar.2006.07.039. Epub 2006 Jul 27. Eur J Pharmacol. 2006. PMID: 16934248 - 3Beta-hydroxysteroids and pregnenolone sulfate inhibit recombinant rat GABA(A) receptor through different channel property.
Wang MD, Rahman M, Zhu D, Johansson IM, Bäckström T. Wang MD, et al. Eur J Pharmacol. 2007 Feb 28;557(2-3):124-31. doi: 10.1016/j.ejphar.2006.11.071. Epub 2006 Dec 12. Eur J Pharmacol. 2007. PMID: 17239367 - Inhibitory Actions of Potentiating Neuroactive Steroids in the Human α1β3γ2L γ-Aminobutyric Acid Type A Receptor.
Pierce SR, Germann AL, Covey DF, Evers AS, Steinbach JH, Akk G. Pierce SR, et al. Mol Pharmacol. 2024 Oct 17;106(5):264-277. doi: 10.1124/molpharm.124.000960. Mol Pharmacol. 2024. PMID: 39214710 - The influence of the membrane on neurosteroid actions at GABA(A) receptors.
Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Akk G, et al. Psychoneuroendocrinology. 2009 Dec;34 Suppl 1:S59-66. doi: 10.1016/j.psyneuen.2009.05.020. Psychoneuroendocrinology. 2009. PMID: 19541427 Free PMC article. Review. - Neurosteroid modulation of recombinant and synaptic GABAA receptors.
Lambert JJ, Harney SC, Belelli D, Peters JA. Lambert JJ, et al. Int Rev Neurobiol. 2001;46:177-205. doi: 10.1016/s0074-7742(01)46063-6. Int Rev Neurobiol. 2001. PMID: 11599300 Review.
Cited by
- Neurosteroid Binding and Actions on GABAA Receptors.
Sugasawa Y. Sugasawa Y. Juntendo Iji Zasshi. 2024 May 24;70(3):239-244. doi: 10.14789/jmj.JMJ24-0002-R. eCollection 2024. Juntendo Iji Zasshi. 2024. PMID: 39429691 Free PMC article. Review. - Modifications of neuroactive steroid levels in an experimental model of nigrostriatal degeneration: potential relevance to the pathophysiology of Parkinson's disease.
Melcangi RC, Caruso D, Levandis G, Abbiati F, Armentero MT, Blandini F. Melcangi RC, et al. J Mol Neurosci. 2012 Jan;46(1):177-83. doi: 10.1007/s12031-011-9570-y. Epub 2011 Jun 14. J Mol Neurosci. 2012. PMID: 21671084 - Neuroactive steroids alphaxalone and CDNC24 are effective hypnotics and potentiators of GABAA currents, but are not neurotoxic to the developing rat brain.
Tesic V, Joksimovic SM, Quillinan N, Krishnan K, Covey DF, Todorovic SM, Jevtovic-Todorovic V. Tesic V, et al. Br J Anaesth. 2020 May;124(5):603-613. doi: 10.1016/j.bja.2020.01.013. Epub 2020 Mar 6. Br J Anaesth. 2020. PMID: 32151384 Free PMC article. - Inhibition of CaV3.2 T-type calcium channels in peripheral sensory neurons contributes to analgesic properties of epipregnanolone.
Ayoola C, Hwang SM, Hong SJ, Rose KE, Boyd C, Bozic N, Park JY, Osuru HP, DiGruccio MR, Covey DF, Jevtovic-Todorovic V, Todorovic SM. Ayoola C, et al. Psychopharmacology (Berl). 2014 Sep;231(17):3503-3515. doi: 10.1007/s00213-014-3588-0. Epub 2014 May 7. Psychopharmacology (Berl). 2014. PMID: 24800894 Free PMC article. - Pregnenolone sulfate analogues differentially modulate GABAA receptor closed/desensitised states.
Mortensen M, Xu Y, Shehata MA, Krall J, Ernst M, Frølund B, Smart TG. Mortensen M, et al. Br J Pharmacol. 2023 Oct;180(19):2482-2499. doi: 10.1111/bph.16143. Epub 2023 Jun 2. Br J Pharmacol. 2023. PMID: 37194503 Free PMC article.
References
- Covey DF, Evers AS, Mennerick S, Zorumski CF, Purdy RH. Recent developments in structure-activity relationships for steroid modulators of GABAA receptors. Brain Res Brain Res Rev. 2001;37:91–97. - PubMed
- Demirgoren S, Majewska MD, Spivak CE, London ED. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience. 1991;45:127–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS040488/NS/NINDS NIH HHS/United States
- MH45493/MH/NIMH NIH HHS/United States
- P01 GM047969/GM/NIGMS NIH HHS/United States
- AA12952/AA/NIAAA NIH HHS/United States
- NS40488/NS/NINDS NIH HHS/United States
- T32 NS007205/NS/NINDS NIH HHS/United States
- R01 AA012952/AA/NIAAA NIH HHS/United States
- T32-NS07205-20/NS/NINDS NIH HHS/United States
- R01 MH045493/MH/NIMH NIH HHS/United States
- GM47969/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources