Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons - PubMed (original) (raw)
Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons
Jakob Wolfart et al. J Neurosci. 2002.
Erratum in
- J Neurosci 2002 June 15;22(12):5250
Abstract
Dopaminergic midbrain (DA) neurons display two principal activity patterns in vivo, single-spike and burst firing, the latter coding for reward-related events. We have shown recently that the small-conductance calcium-activated potassium channel SK3 controls pacemaker frequency and precision in DA neurons of the substantia nigra (SN), and previous studies have implicated SK channels in the transition to burst firing. To identify the upstream calcium sources for SK channel activation in DA SN neurons, we studied the sensitivity of SK channel-mediated afterhyperpolarization (AHP) currents to inhibitors of different types of voltage-gated calcium channels in perforated patch-clamp recordings. Cobalt-sensitive AHP currents were not affected by L-type and P/Q-type calcium channel inhibitors and were reduced slightly (26%) by the N-type channel inhibitor omega-conotoxin-GVIA. In contrast, AHP currents were blocked substantially (85-94%) by micromolar concentrations of nickel (IC50, 33.75 microm) and mibefradil (IC50, 4.83 microm), indistinguishable from the nickel and mibefradil sensitivities of T-type calcium currents (IC50 values, 33.86 and 4.59 microm, respectively). These results indicate that SK channels are activated selectively via T-type calcium channels in DA SN neurons. Consequently, SK currents displayed use-dependent inactivation with similar time constants when compared with those of T-type calcium currents and generated a transient rebound inhibition. Both SK and T-type channels were essential for the stability of spontaneous pacemaker activity, and, in some DA SN neurons, T-type channel inhibition was sufficient to induce intrinsic burst firing. The functional coupling of SK to T-type channels has important implications for the temporal integration of synaptic input and might help to understand how DA neurons switch between pacemaker and burst-firing modes in vivo.
Figures
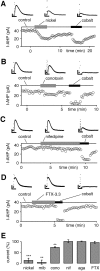
Fig. 1.
Sensitivity of hybrid-clamp-evoked SK channel-mediated AHP currents (I-AHP) to inhibitors of Cav channels recorded in the perforated whole-cell configuration. A, Low micromolar concentrations (100 μ
m
) of nickel (T-type) reversibly inhibited most of the cobalt-sensitive I-AHP. B, ω-Conotoxin-GVIA (conotoxin; 1 μ
m
) reversibly reduced a minor part of the cobalt-sensitive I-AHP. C, Nifedipine (10 μ
m
) did not affect I-AHPs.D, FTX-3.3 (1 μ
m
) had no effect on I-AHPs.E, The summary of experiments in _A–D_shows that cobalt-sensitive I-AHPs were activated preferentially via calcium channels sensitive to low micromolar nickel (100 μ
m;
85 ± 9%; n = 13; ∗∗∗p < 0.0005) and mibefradil (mib; 10 μ
m;
94 ± 10%; n = 6; ∗∗p < 0.005), whereas only a small component was sensitive to 1 μ
m
ω-conotoxin-GVIA (cono; 26 ± 3%; n = 4; ∗∗p < 0.005). Nifedipine (nif; 10 μ
m
) did not affect I-AHPs (residual current, 102 ± 6%; n = 6; p > 0.05). Similarly, 1 μ
m
FTX-3.3 (FTX; residual current, 97 ± 5%; n = 5; p > 0.05) and 0.1 μ
m
agatoxin-TK (aga; residual current, 101 ± 1%; n = 3; p > 0.05) had no effect on I-AHPs. Current amplitudes were normalized to cobalt-sensitive I-AHP in each individual experiment except for mibefradil, in which the mean value of cobalt block was used (1 m
m;
residual current, 29 ± 4%; _n_= 29). Calibration: A–D, 0.2 sec, 10 pA.
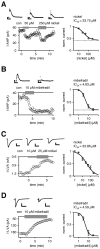
Fig. 2.
SK-mediated AHP and T-type-mediated low voltage-activated calcium currents (I-LVA; see Results) possessed almost identical nickel and mibefradil sensitivities in dopaminergic neurons. A, Nickel reduced AHP currents (I-AHP; evoked by 20 msec hybrid-clamp depolarizations) in a concentration-dependent manner. The mean dose–response relationship for nickel I-AHP inhibition was described by a Hill function, with an IC50 of 33.75 μ
m
, a Hill coefficient of 1.30 (n = 19), and a relative fitted residual I-AHP component (42%; dotted line).B, Mibefradil inhibited a major component of the I-AHP irreversibly. The mean dose–response relationship for mibefradil I-AHP inhibition was described by a Hill function, with an IC50 of 4.83 μ
m
(Hill coefficient, 2.16; n = 10) and a relative fitted residual I-AHP component of 20% (dotted line).C, T-type-mediated LVA currents evoked by depolarizations to −50 mV from a holding potential of −100 mV were recorded by using standard whole-cell recordings (see Results and Materials and Methods). Nickel reduced the I-LVA in a concentration-dependent manner. The mean dose–response relationship for nickel I-LVA inhibition was described by a Hill function, with an IC50 of 33.86 μ
m
(Hill coefficient, 0.85;n = 16). D, Mibefradil inhibited the I-LVA irreversibly. The mean dose–response for mibefradil I-LVA inhibition was described by a Hill function, with an IC50of 4.59 μ
m
(Hill coefficient, 2.27; _n_= 14). Calibration: A, B, 0.2 sec, 10 pA;C, 0.2 sec, 300 pA; D, 0.2 sec, 60 pA.
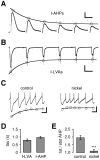
Fig. 3.
Use-dependent inactivation of SK and T-type currents displayed similar kinetics. A, AHP currents (I-AHPs) evoked with hybrid-clamp depolarizations (100 msec, +60 mV) at a frequency of 1 Hz by using the standard whole-cell configuration (recording potential, −80 mV). Successive AHP currents decreased, reaching a steady-state level at 38% of the initial amplitude. The time constant of cumulative inactivation was 1.26 sec.B, Recording of T-type-mediated low voltage-activated calcium currents (I-LVA; see Results) evoked by the same voltage pulse protocol as in A, using the standard whole-cell configuration and calcium channel recording solutions. Successive activation at 1 Hz led to a decrease of I-LVAs, reaching a steady-state level of 42%. The time constant of use-dependent inactivation was 0.77 sec. C, Perforated current-clamp recording of a train of APs evoked by injections of 10 pA for 4 sec from a hyperpolarized membrane potential of −80 mV. At the onset of depolarization the AHPs were large but decreased with successive APs. Application of nickel (250 μ
m
) decreased the AHP amplitudes and abolished the effect of cumulative inactivation. Note that the control rate of cumulative AHP inactivation (τ = 1.10 sec) was similar to the time constants determined for I-AHPs and I-LVAs. D, Time constants (τ) of cumulative inactivation determined by experiments in A and_B_. LVA and AHP currents both had cumulative inactivation time constants in the range of 1 sec: I-LVA, 0.80 ± 0.07 sec (n = 10); I-AHP, 0.98 ± 0.06 sec (n = 10; p > 0.05).E, The summary of experiments in D shows that AHPs were reduced twofold under control conditions (2.0 ± 0.1; n = 8), whereas the effect was abolished by nickel application (1.1 ± 0.1; n = 8; ∗∗∗p < 0.0005). Calibration:A, 0.5 sec, 50 pA; B, 0.5 sec, 200 pA;C, 0.5 sec, 10 mV.
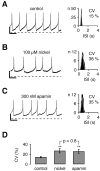
Fig. 4.
Nickel-sensitive T-type and apamin-sensitive SK channels maintained the high precision of pacemaker spiking in dopaminergic neurons. A–C, Perforated current-clamp recordings during control (A), 100 μ
m
nickel (B), and 300 n
m
apamin (C) application.Left panels show a 4 sec recording trace representative of a 5 min recording for each condition. Interspike interval (ISI) frequency distributions are displayed in the right panels for each recording. As a measure of pacemaker precision the coefficient of variation (CV) was calculated from the Gaussian fit of ISI histograms. APs were truncated at −20 mV. A, During control conditions pacemaker spiking was relatively regular, with a CV of 15%. B, Application of 100 μ
m
nickel reversibly rendered pacemaker spiking to be more irregular (CV of 38%). C, Application of 300 n
m
apamin did increase the irregularity of pacemaker spiking to a similar degree (CV of 35%). D, The summary of experiments in_A–C_ shows that nickel (100 μ
m
) and apamin (300 n
m
) decreased the pacemaker precision (∗∗p < 0.005, respectively) to a similar extent (_p_ > 0.8). Mean CVs: control, 14 ± 2% (n = 11); nickel, 27 ± 5% (n = 11); apamin, 26 ± 4% (n = 11). Calibration: A–C, 0.5 sec, 10 mV. Dotted line in A–C, −50 mV.
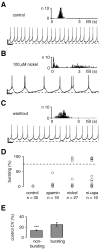
Fig. 5.
Inhibition of T-type channels evoked bursting in a subpopulation of dopaminergic midbrain neurons. A–C, Perforated current-clamp recordings during control (A), nickel (B), and washout (C) conditions. A 20 sec recording trace representative of a 5 min recording is shown for each condition. As a measure of pacemaker precision the coefficient of variation (CV) was calculated from Gaussian fits of ISI histograms (insets).A, No bursting (0%) was detected during control application. Note that this neuron showed pacemaker spiking at the lower end of firing precision (CV of 20%; compare with Fig. 4).B, Application of 100 μ
m
nickel switched the firing pattern from pacemaker to bursting, with two to three closely spaced APs alternating with long inter-burst intervals. C, With the washout of nickel the firing pattern returned to (irregular) pacemaker spiking.D, Firing patterns during perforated patch-clamp recordings were assessed by a burst evaluation program (spikes/burst per trace = bursting in percentage values; e.g., 85% bursting for the recording shown in B). Under control conditions the bursting value was 0% (n = 30). Application of 300 n
m
apamin did not change the bursting value (3 ± 2%;n = 19) significantly, although one cell showed an increased value (45%) because of short periods of “burst-like” pattern. Inhibition of T-type channels with 100 μ
m
nickel significantly increased the bursting value to 12 ± 6% (n = 27; p < 0.05), and three cells displayed bursting values above 84%. The combination of nickel and apamin application (ni+apa_) was most effective in switching from pacemaker to bursting behavior, increasing the mean bursting value to 34 ± 10% (n = 16;p < 0.0005), with five neurons reaching bursting values of >86%. E, Differential effects of T-type channel inhibition on firing patterns were associated to pacemaker precision under control conditions. Control CV values were correlated with the effect of nickel and apamin application on firing patterns of respective cells. Neurons that were converted to bursting had significantly higher CV values (25 ± 4%; n = 6) compared with cells that became irregular with nickel (or nickel + apamin) application (14 ± 1%; n = 22; ∗∗∗_p < 0.0005). Calibration:A–C, 1 sec, 10 mV. Dotted lines in_A–C_, −50 mV. Dotted line in_D_, 75% bursting.
Similar articles
- Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons.
Wolfart J, Neuhoff H, Franz O, Roeper J. Wolfart J, et al. J Neurosci. 2001 May 15;21(10):3443-56. doi: 10.1523/JNEUROSCI.21-10-03443.2001. J Neurosci. 2001. PMID: 11331374 Free PMC article. - Interactions between calcium channels and SK channels in midbrain dopamine neurons and their impact on pacemaker regularity: Contrasting roles of N- and L-type channels.
de Vrind V, Scuvée-Moreau J, Drion G, Hmaied C, Philippart F, Engel D, Seutin V. de Vrind V, et al. Eur J Pharmacol. 2016 Oct 5;788:274-279. doi: 10.1016/j.ejphar.2016.06.046. Epub 2016 Jun 27. Eur J Pharmacol. 2016. PMID: 27364758 - Small conductance Ca2+-activated K+ channels as targets of CNS drug development.
Blank T, Nijholt I, Kye MJ, Spiess J. Blank T, et al. Curr Drug Targets CNS Neurol Disord. 2004 Jun;3(3):161-7. doi: 10.2174/1568007043337472. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15180477 Review. - Matching molecules to function: neuronal Ca2+-activated K+ channels and afterhyperpolarizations.
Stocker M, Hirzel K, D'hoedt D, Pedarzani P. Stocker M, et al. Toxicon. 2004 Jun 15;43(8):933-49. doi: 10.1016/j.toxicon.2003.12.009. Toxicon. 2004. PMID: 15208027 Review.
Cited by
- Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons.
Womack MD, Chevez C, Khodakhah K. Womack MD, et al. J Neurosci. 2004 Oct 6;24(40):8818-22. doi: 10.1523/JNEUROSCI.2915-04.2004. J Neurosci. 2004. PMID: 15470147 Free PMC article. - Functional diversity of ventral midbrain dopamine and GABAergic neurons.
Korotkova TM, Ponomarenko AA, Brown RE, Haas HL. Korotkova TM, et al. Mol Neurobiol. 2004 Jun;29(3):243-59. doi: 10.1385/MN:29:3:243. Mol Neurobiol. 2004. PMID: 15181237 Review. - Functional coupling between large-conductance potassium channels and Cav3.2 voltage-dependent calcium channels participates in prostate cancer cell growth.
Gackière F, Warnier M, Katsogiannou M, Derouiche S, Delcourt P, Dewailly E, Slomianny C, Humez S, Prevarskaya N, Roudbaraki M, Mariot P. Gackière F, et al. Biol Open. 2013 Jul 26;2(9):941-51. doi: 10.1242/bio.20135215. eCollection 2013. Biol Open. 2013. PMID: 24143281 Free PMC article. - Dopamine Neurons Change the Type of Excitability in Response to Stimuli.
Morozova EO, Zakharov D, Gutkin BS, Lapish CC, Kuznetsov A. Morozova EO, et al. PLoS Comput Biol. 2016 Dec 8;12(12):e1005233. doi: 10.1371/journal.pcbi.1005233. eCollection 2016 Dec. PLoS Comput Biol. 2016. PMID: 27930673 Free PMC article. - Enhanced Sensitivity to Hyperpolarizing Inhibition in Mesoaccumbal Relative to Nigrostriatal Dopamine Neuron Subpopulations.
Tarfa RA, Evans RC, Khaliq ZM. Tarfa RA, et al. J Neurosci. 2017 Mar 22;37(12):3311-3330. doi: 10.1523/JNEUROSCI.2969-16.2017. Epub 2017 Feb 20. J Neurosci. 2017. PMID: 28219982 Free PMC article.
References
- Bezprozvanny I, Tsien RW. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967). Mol Pharmacol. 1995;48:540–549. - PubMed
- Blatz A, Magleby K (1987) Calcium-activated potassium channels. Trends Neurosci 463–467.
- Bond CT, Maylie J, Adelman JP. Small-conductance calcium-activated potassium channels. Ann NY Acad Sci. 1999;868:370–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources