Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions - PubMed (original) (raw)
Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions
Andrea B Huber et al. J Neurosci. 2002.
Abstract
Nogo-A is a neurite growth inhibitor involved in regenerative failure and restriction of structural plasticity in the adult CNS. Three major protein products (Nogo-A, -B, and -C) are derived from the nogo gene. Here we describe the embryonic and postnatal expression of the three Nogo isoforms in the rat by in situ hybridization and immunohistochemistry. Northern and Western blot analysis indicated that Nogo-A is predominantly expressed in the nervous system with lower levels also present in testis and heart. In CNS myelin, confocal and immunoelectron microscopy revealed that Nogo-A is expressed in oligodendrocyte cell bodies and processes and localized in the innermost adaxonal and outermost myelin membranes. Additionally, we find Nogo-A to be expressed by projection neurons, in particular during development, and by postmitotic cells in the developing cortex, spinal cord, and cerebellum. The expression levels of Nogo-A/B were not changed significantly after traumatic lesions to the cortex or spinal cord. Nogo-B showed widespread expression in the central and peripheral nervous systems and other peripheral tissues. Nogo-C was mainly found in skeletal muscle, but brain and heart were also found to express this isoform. The localization of Nogo-A in oligodendrocytes fits well with its role as a myelin-associated inhibitor of regenerative fiber growth and structural plasticity. However, expression of Nogo-A in other tissues and, in particular, in neurons and the widespread expression of the two shorter isoforms, Nogo-B and -C, suggest that the Nogo family of proteins might have function(s) additional to the neurite growth-inhibitory activity.
Figures
Fig. 1.
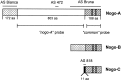
Nogo isoforms, probes, and antisera. The three_nogo_ transcripts with the common region (hatched), Nogo-A-specific region (white), Nogo-A/B-specific N terminus (dotted), and Nogo-C-specific N terminus (black, 11 aa) are shown. Two long hydrophobic stretches (35 and 36 aa) in the region common to all three isoforms, serving as potential transmembrane domains, are marked (gray). Riboprobes used for in situ hybridization and Northern blot are shown:nogo-A and common probes. AS 472 and AS 818 were raised against peptides recognizing Nogo-A and -C, respectively. AS Bianca was raised against the Nogo-A/B-specific N terminus, and AS Bruna was raised against a bacterial recombinant protein including the common part present in all three Nogo isoforms and the C-terminal portion of the Nogo-A-specific region. Note that Nogo-B has no unique sequence.
Fig. 2.
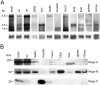
Northern and Western blot analysis.A, Northern hybridization with the C-terminal_common_ probe on mRNA of adult rat tissues revealed_nogo-A_ (4.6 kb) to be strongly expressed in brain, spinal cord (sc), and optic nerve (on). Low levels of nogo-A mRNA were found in DRG, sciatic nerve (scn), testis, and heart. Note that for DRG and sciatic nerve, less mRNA was loaded than for the other tissues.nogo-B mRNA (2.6 kb) was high in the CNS but was also detected in DRG, sciatic nerve, lung, and kidney and at lower level in testis, liver, and spleen. Strong expression of nogo-C(1.7 kb) was observed in spinal cord, brain, optic nerve, and skeletal muscle. In sciatic nerve, heart, liver, spleen, and kidney, expression was lower. For loading control, the blot was reprobed with a glyceraldehyde-3-phosphate dehydrogenase riboprobe. B, In the adult rat, Nogo-A (190 kDa) was strongly expressed in brain and spinal cord, as revealed by AS 472 (and AS Bruna and AS Bianca; data not shown). Apart from the nervous system, Nogo-A was only expressed in detectable amounts in testis and heart. The band present in liver (asterisk) was also detected by preimmune sera and secondary antibody only and represents therefore an unspecific signal. AS Bianca showed expression of Nogo-B (55 kDa) in brain, spinal cord, testis, heart, lung, liver, spleen, and kidney. AS 818 detected a strong Nogo-C band at 25 kDa in skeletal muscle. The smallest Nogo isoform was also present in brain and heart, 40 and 5%, respectively, of the amount present in skeletal muscle, as revealed by densitometric analysis on Western blots with the same amount of total protein loaded.
Fig. 3.
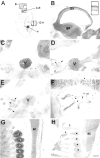
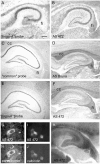
Nogo expression in the E16 rat embryo.In situ hybridization with the _nogo-A_probe revealed strong expression in the mantle layer of postmitotic neurons in the developing forebrain cortex (B,inset). In the trigeminal ganglion (C, D;V), high levels of Nogo-A were detected (C, nogo-A probe; D, AS 472). Nogo-A protein was also found in the trigeminal nerve fibers, extraocular muscles (arrows), and optic nerve (arrowhead). Note that Nogo-C (AS 818; E) was only present in extraocular muscles (arrows) but not in the trigeminal ganglion or optic nerve. Strong expression of Nogo-C was found with AS 818 in intestinal epithelium (F,arrows). G, H, Nogo-A mRNA (G) and protein (H) expression in the spinal cord (sc) and DRG (asterisks). Again, protein expression revealed by AS 472 was also found in nerve fibers (H, arrows), whereas mRNA expression (nogo-A probe) was restricted to neuronal cell bodies in the DRG and spinal cord. The level of mRNA expression in DRG was higher than in the spinal cord; however, protein levels were comparable. Scale bar: B–E, G, H, 550 μm;F, B, inset, 140 μm.
Fig. 4.
Nogo-A expression in the spinal cord and dorsal root ganglia. In situ hybridization with the_nogo-A_ probe showed the presence of this transcript in neuronal subtypes of the spinal cord, DRG, and sympathetic ganglia (sg) at E14 (A) and E16 (B). In the adult, nogo-A mRNA was found in oligodendrocyte cell bodies in the white matter (C, arrowheads). The corresponding immunohistochemistry with AS 472 (E, I) revealed Nogo-A expression mainly in the myelinated areas of the spinal cord and very intensely in oligodendrocyte cell bodies (E, I,arrowheads). Less strongly myelinated areas such as the region of the corticospinal tract (E,arrow) were also stained less. Fixation of the tissue with ethanol and acetic acid (A, B, E, G, I) revealed the Nogo-A localized to myelin and oligodendrocyte cell bodies. Neuronal Nogo-A was visualized by fixation with methanol (F, H, J). Spinal neurons in the gray matter were expressing nogo-A mRNA (C) and Nogo-A protein (H, high magnification of motor neurons). Strong Nogo-A expression was found in adult DRG neurons and their neurites (F, arrows), whereas the neurites were not stained with the nogo-A probe (D, arrows). mAb IN-1 strongly stained white matter and oligodendrocyte cell bodies (G) as well as motor neurons (methanol fixation; H). Scale bar: A, C, E, G, 460 μm; B, 275 μm; D, F, H–J, 140 μm.
Fig. 5.
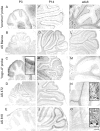
Nogo-A, -B, and -C expression in the neocortex.A–E, In situ hybridizations with the_common_ probe. F, AS Bruna;G, “nogo-A” probe; H, AS 472; I, AS 818; J, Nissl staining. In E19 (A), a strong nogo-A/B signal was observed in the matrix layer (ml), mantle layer (mal), and cortical plate (cp). Also at birth (B), strong expression in cortical plate and subplate was found, which was persisting in P3 (C) and P5 (D). Neurons in layer V were strongly expressing_nogo-A/B_ in P3, whereas layer VI was only weakly stained. In the adult (E–J), neurons in layer IV of the cortex were stained weaker for Nogo-A/B than neurons in layers II and III and V and VI. Very low levels of Nogo-C protein were detected with AS 818 (I). Image width:A–D, 210 μm; E–J, 420 μm.
Fig. 6.
Nogo-A/B expression in the hippocampus. At birth, expression of both Nogo-A mRNA (A) and protein (B) was seen in pyramidal cells of hippocampal regions CA1–CA4, whereas weaker staining was found in the granule neurons of the dentate gyrus (A, nogo-A probe;B, AS 472). Nogo-A was also found in the fimbria (fi). In the adult hippocampus, Nogo-A/B was expressed in pyramidal cells of CA1–CA4 (C, _common_probe; D, AS Bruna; E,nogo-A probe; F, AS 472). The distinctly weaker signals of these neurons in E, _F_indicate that the predominant form might be Nogo-B. Note the high Nogo-A protein levels in the myelinated fiber tracts of the corpus callosum (cc) and fimbria–fornix (fi). A histoblot probed with AS 472 revealed expression of Nogo-A also in hippocampal fiber layers (K). Black, High Nogo-A expression; whitem low Nogo-A expression. Nogo-A (G, I, AS 472) was expressed in parvalbumin-positive (H) and calbindin-positive (J) interneurons in the hilus. Scale bar:A, B, 275 μm; C–F, K, 550 μm;G, H, 10 μm; I, J, 20 μm.
Fig. 7.
Nogo expression in the cerebellum. At P3 (A–E), Nogo-A/B was expressed in Purkinje cells (A, common probe; B, AS Bianca; C, nogo-A probe;D, AS 472), whereas very little Nogo-C was found in these neurons (E, AS 818). Nogo-C revealed by immunohistochemistry with AS 818 was found in Purkinje cells in P14 and adult (J, O). Nogo-A-positive neurons in the premigratory layer of the EGL were more strongly stained than the outermost, proliferative sublayer (C, inset). Nogo-A/B expression remained high in oligodendrocytes in the white matter and in oligodendrocytes in the granule cell layer in P14 and adult. Neurons in the deep cerebellar nuclei were strongly expressing Nogo-A/B at all time points. Sections stained with AS Bianca (B, G, L) and AS 472 (D, I, N) were fixed with EGTA-ethanol and acetic acid, revealing the prominent expression of Nogo-A/B in white matter, Purkinje cells, and their dendrites. Scale bar: 275 μm;insets, 35 μm.
Fig. 8.
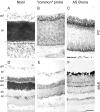
Nogo expression in the retina. At birth (A–C), strong expression of Nogo-A/B mRNA (B, common probe) and protein (C, AS Bruna) in the ganglion cell layer (gcl) as well as the cytoblast layer (cb) was observed. Expression was highest at the interface between the ganglion cell layer and cytoblast layer. The ganglion cells were also Nogo-A/B-positive in the adult (D–F), and both inner and outer nuclear layers (inl, onl) were expressing mRNA (E, common probe) and protein (F, AS Bruna). Nogo-A/B protein seemed to be targeted to neurites, because the inner and outer plexiform layers (ipl, opl) were positive for AS Bruna (F). A, D, Nissl staining. Scale bar, 70 μm.
Fig. 9.
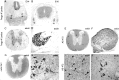
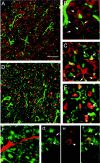
Localization of Nogo-A in CNS white matter by confocal microscopic analysis. A, No colocalization of Nogo-A (shown by AS 472 in green) was found with MBP (red), which is a major constituent of compact myelin. Nogo-A was expressed in oligodendrocyte cell bodies, their processes, and the inner loop (B, C,arrowheads) and outer loop (B, C,arrows) of the myelin sheath. The fixation protocol required to demonstrate MBP expression lowered the Nogo-A signal in the inner loop of the myelin membrane, whereas the different protocol used for MAG and MOG immunohistochemistry showed strong expression of Nogo-A in the inner and outer loops of the myelin sheath.E, Nogo-A (AS 472, green) is expressed in the outer loop (arrows) of the myelin sheath and was found to colocalize there with MOG (red).G, A strong Nogo-A signal (AS 472, green) was also found in the inner loop (arrowheads), where MAG (H, red) is expressed. C, D, Some Nogo-A (AS 472, green) was also present in axons, as was demonstrated by colocalization (yellow) of AS 472 with an antibody against neurofilament (red). F, Nogo-A (AS 472,green) was not present in astrocyte processes stained by an antibody against GFAP (red). Scale bar: A, C, 85 μm; B, D–I, 45 μm.
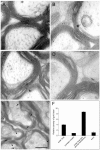
Fig. 10.
Localization of Nogo-A in the optic nerve by immunoelectron microscopy. Nogo-A was detected on optic nerve ultrathin sections by immunogold electron microscopy. A, B, AS 472 gold grains (arrows) were found at the inner loop and less frequently at the outer loop of the myelin sheath. Expression of MAG (C, arrowhead) was detected in the innermost loop, and MOG (D,arrowhead) immunoreactivity was detected in the outer loop. E, Double labeling for Nogo-A (small grains;arrows) and MAG (large grains;arrowheads) confirmed this distribution.F, Quantification and statistical analysis of the distribution of Nogo-A protein in adult rat optic nerve. Scale bar:A–D, 0.25 μm; E, 0.5 μm.
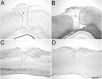
Fig. 11.
Expression of Nogo mRNA and protein is unchanged after traumatic CNS injury. A, In situ hybridization with common Nogo probe 4 d after cortical lesion. B, Corresponding immunohistochemistry (AS Bruna) on an adjacent section. A slight upregulation of Nogo-A/B in cortical neurons in the vicinity of the injury was observed at this time point but not at any other time point or in spinal cord. Note the Nogo-free lesion area and lesion borders.C, In situ hybridization with the_common_ probe. D, Immunohistochemistry with AS Bruna 4 weeks after spinal lesion. No detectable upregulation or downregulation can be observed. The lesion areas are_outlined_ and marked by asterisks. Scale bar, 550 μm.
Similar articles
- Activity-induced and developmental downregulation of the Nogo receptor.
Josephson A, Trifunovski A, Schéele C, Widenfalk J, Wahlestedt C, Brené S, Olson L, Spenger C. Josephson A, et al. Cell Tissue Res. 2003 Mar;311(3):333-42. doi: 10.1007/s00441-002-0695-8. Epub 2003 Jan 31. Cell Tissue Res. 2003. PMID: 12658441 - NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury.
Josephson A, Widenfalk J, Widmer HW, Olson L, Spenger C. Josephson A, et al. Exp Neurol. 2001 Jun;169(2):319-28. doi: 10.1006/exnr.2001.7659. Exp Neurol. 2001. PMID: 11358445 - Nogo-receptor gene activity: cellular localization and developmental regulation of mRNA in mice and humans.
Josephson A, Trifunovski A, Widmer HR, Widenfalk J, Olson L, Spenger C. Josephson A, et al. J Comp Neurol. 2002 Nov 18;453(3):292-304. doi: 10.1002/cne.10408. J Comp Neurol. 2002. PMID: 12378589 - The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.
Hunt D, Coffin RS, Anderson PN. Hunt D, et al. J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review. - [Inhibitory proteins against axon regeneration in the central nervous system].
Hu JG, Lu PH, Xu XM. Hu JG, et al. Sheng Li Ke Xue Jin Zhan. 2004 Oct;35(4):311-4. Sheng Li Ke Xue Jin Zhan. 2004. PMID: 15727207 Review. Chinese.
Cited by
- Reticulon 4B (Nogo-B) facilitates hepatocyte proliferation and liver regeneration in mice.
Gao L, Utsumi T, Tashiro K, Liu B, Zhang D, Swenson ES, Iwakiri Y. Gao L, et al. Hepatology. 2013 May;57(5):1992-2003. doi: 10.1002/hep.26235. Epub 2013 Mar 19. Hepatology. 2013. PMID: 23299899 Free PMC article. - A Network Pharmacology Analysis to Explore the Effect of Astragali Radix-Radix Angelica Sinensis on Traumatic Brain Injury.
Xie G, Peng W, Li P, Xia Z, Zhong Y, He F, Tulake Y, Feng D, Wang Y, Xing Z. Xie G, et al. Biomed Res Int. 2018 Nov 25;2018:3951783. doi: 10.1155/2018/3951783. eCollection 2018. Biomed Res Int. 2018. PMID: 30596090 Free PMC article. - Soluble Nogo receptor down-regulates expression of neuronal Nogo-A to enhance axonal regeneration.
Peng X, Zhou Z, Hu J, Fink DJ, Mata M. Peng X, et al. J Biol Chem. 2010 Jan 22;285(4):2783-95. doi: 10.1074/jbc.M109.046425. Epub 2009 Nov 9. J Biol Chem. 2010. PMID: 19901030 Free PMC article. - Constitutive genetic deletion of the growth regulator Nogo-A induces schizophrenia-related endophenotypes.
Willi R, Weinmann O, Winter C, Klein J, Sohr R, Schnell L, Yee BK, Feldon J, Schwab ME. Willi R, et al. J Neurosci. 2010 Jan 13;30(2):556-67. doi: 10.1523/JNEUROSCI.4393-09.2010. J Neurosci. 2010. PMID: 20071518 Free PMC article. - Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity.
Maier IC, Schwab ME. Maier IC, et al. Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1611-34. doi: 10.1098/rstb.2006.1890. Philos Trans R Soc Lond B Biol Sci. 2006. PMID: 16939978 Free PMC article. Review.
References
- Alcantara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin-1 acts as an attractive of repulsive cue for distinct migration neurons during the development of the cerebellar system. Development. 2000;127:1359–1372. - PubMed
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972a;145:353–397. - PubMed
- Altman J. Postnatal development of the cerebellar cortex in the rat. III. Maturation of the components of the granular layer. J Comp Neurol. 1972b;145:465–513. - PubMed
- Bartholdi D, Schwab ME. Oligodendroglial reaction following spinal cord injury in rat: transient upregulation of MBP mRNA. Glia. 1998;23:278–284. - PubMed
- Benke D, Wenzel A, Scheuer L, Fritschy JM, Mohler H. Immunobiological characterization of the NMDA-receptor subunit NR1 in the developing and adult rat brain. J Recept Signal Transduct Res. 1995;15:393–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources