Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats - PubMed (original) (raw)
Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats
Michelle M Adams et al. J Neurosci. 2002.
Abstract
Estrogen replacement increases both the number of dendritic spines and the density of axospinous synapses in the hippocampal CA1 region in young rats, yet this is attenuated in aged rats. The estrogen receptor-alpha (ER-alpha) is localized within select spines of CA1 pyramidal cells in young animals and thus may be involved locally in this process. The present study investigated the effects of estrogen on the ultrastructural distribution of ER-alpha in the CA1 of young (3-4 months) and aged (22-23 months) Sprague Dawley rats using postembedding immunogold electron microscopy. Within dendritic spines, most ER-alpha immunoreactivity (IR) was seen in plasmalemmal and cytoplasmic regions of spine heads, with a smaller proportion within 60 nm of the postsynaptic density. In presynaptic terminals, ER-alpha-IR was clustered and often associated with synaptic vesicles. Significant effects of both aging and estrogen were observed. Quantitative analysis revealed that nonsynaptic pools of ER-alpha-IR within the presynaptic and postsynaptic compartments were decreased (35 and 27%, respectively) in the young estrogen-replaced animals compared with those that received vehicle. Such localized regulation of ER-alpha in response to circulating estrogen levels might directly affect synaptic signaling in CA1 pyramidal cells. No estrogen treatment-related differences were observed in the aged animals. However, 50% fewer spines contained ER-alpha in the aged compared with young hippocampus. These data suggest that the decreased responsiveness of hippocampal synapses to estrogen in aged animals may result from age-related decrements in ER-alpha levels and its subcellular localization vis-à-vis the synapse. Such a role for spinous ER-alpha has important implications for age-related attenuation of estrogen-induced hippocampal plasticity.
Figures
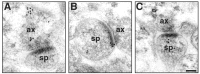
Fig. 1.
Distribution of ER-α-IR within axospinous synapses in the stratum radiatum of the hippocampal CA1 region. Gold particles were observed to be localized both presynaptically (A) and postsynaptically (B, C). In the axon terminal (A), gold particles were associated with small synaptic vesicles. In dendritic spines, gold particles were found within the head of the spine (B, C) and affiliated within the postsynaptic density. Particles within the postsynaptic density were usually found in the more lateral portion.ax, Axon; sp, spine. Scale bar, 100 nm.
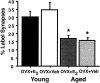
Fig. 2.
Percentage of ER-α-IR-labeled synapses in young and aged rats. The graphs illustrate that the percentage of ER-α-IR synapses decreases (50%) with age (*p < 0.0001); however, there is no effect of estrogen treatment in either group (both _p_ values > 0.31).n = 10 for each age group. OVX, Ovariectomized; E 2, estrogen-treated;Veh, vehicle-treated.
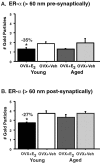
Fig. 3.
Levels of ER-α-IR per synapse in young and aged vehicle-treated (Veh) and estrogen-treated (E 2) rats. The amount of ER-α-IR particles per synapse in young rats significantly decreases with estrogen treatment compared with vehicle both presynaptically (A) and postsynaptically (B) (*both p values < 0.05). No differences were observed in the estrogen- and vehicle-treated aged groups (both _p_ values > 0.16).n = 5 for each treatment group. OVX, Ovariectomized.
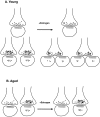
Fig. 4.
Schematic diagram illustrating the proposed mechanisms of estrogen-induced plasticity of ER-α in young (A) and aged (B) animals. Estrogen treatment either decreases the amount of ER-α per synapse in young animals or leads to a redistribution of the receptor resulting from the formation of new dendritic spines and alterations in presynaptic terminal shape. ER-α levels are unchanged in aged animals in both presynaptic and postsynaptic compartments, but there is an overall decrease in the percentage of terminals that express ER-α-IR. Small black dots represent immunogold particles labeling ER-α, open circles represent synaptic vesicles, and the gray zones represent the postsynaptic density.
Similar articles
- Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in CA1 region of female rat hippocampus.
Yildirim M, Janssen WG, Tabori NE, Adams MM, Yuen GS, Akama KT, McEwen BS, Milner TA, Morrison JH. Yildirim M, et al. Neuroscience. 2008 Mar 18;152(2):360-70. doi: 10.1016/j.neuroscience.2008.01.004. Epub 2008 Jan 12. Neuroscience. 2008. PMID: 18294775 Free PMC article. - Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus.
Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Towart LA, et al. J Comp Neurol. 2003 Sep 1;463(4):390-401. doi: 10.1002/cne.10753. J Comp Neurol. 2003. PMID: 12836175 - Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus.
Tippens AL, Pare JF, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A. Tippens AL, et al. J Comp Neurol. 2008 Feb 1;506(4):569-83. doi: 10.1002/cne.21567. J Comp Neurol. 2008. PMID: 18067152 - Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging.
Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH. Bailey ME, et al. Neuroscience. 2011 Sep 15;191:148-58. doi: 10.1016/j.neuroscience.2011.05.045. Epub 2011 Jun 2. Neuroscience. 2011. PMID: 21664255 Free PMC article. Review. - Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons.
Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto T, Kawato S. Ogiue-Ikeda M, et al. Brain Res Rev. 2008 Mar;57(2):363-75. doi: 10.1016/j.brainresrev.2007.06.010. Epub 2007 Jul 28. Brain Res Rev. 2008. PMID: 17822775 Review.
Cited by
- Sex chromosomes and hormones independently influence healthy brain development but act similarly after cranial radiation.
Yeung J, DeYoung T, Spring S, de Guzman AE, Elder MW, Beauchamp A, Wong CS, Palmert MR, Lerch JP, Nieman BJ. Yeung J, et al. Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2404042121. doi: 10.1073/pnas.2404042121. Epub 2024 Aug 29. Proc Natl Acad Sci U S A. 2024. PMID: 39207735 - The intersection between menopause and depression: overview of research using animal models.
Herrera-Pérez JJ, Hernández-Hernández OT, Flores-Ramos M, Cueto-Escobedo J, Rodríguez-Landa JF, Martínez-Mota L. Herrera-Pérez JJ, et al. Front Psychiatry. 2024 Jul 15;15:1408878. doi: 10.3389/fpsyt.2024.1408878. eCollection 2024. Front Psychiatry. 2024. PMID: 39081530 Free PMC article. Review. - The impact of estradiol on serotonin, glutamate, and dopamine systems.
Bendis PC, Zimmerman S, Onisiforou A, Zanos P, Georgiou P. Bendis PC, et al. Front Neurosci. 2024 Mar 22;18:1348551. doi: 10.3389/fnins.2024.1348551. eCollection 2024. Front Neurosci. 2024. PMID: 38586193 Free PMC article. Review. - TNFR1/p38αMAPK signaling in Nex + supraspinal neurons regulates estrogen-dependent chronic neuropathic pain.
Swanson KA, Nguyen KL, Gupta S, Ricard J, Bethea JR. Swanson KA, et al. Brain Behav Immun. 2024 Jul;119:261-271. doi: 10.1016/j.bbi.2024.03.050. Epub 2024 Apr 1. Brain Behav Immun. 2024. PMID: 38570102 - Increased palmitoylation improves estrogen receptor alpha-dependent hippocampal synaptic deficits in a mouse model of synucleinopathy.
Moors TE, Li S, McCaffery TD, Ho GPH, Bechade PA, Pham LN, Ericsson M, Nuber S. Moors TE, et al. Sci Adv. 2023 Nov 15;9(46):eadj1454. doi: 10.1126/sciadv.adj1454. Epub 2023 Nov 17. Sci Adv. 2023. PMID: 37976363 Free PMC article.
References
- Adams MM, Oung T, Morrison JH, Gore AC. Length of postovariectomy interval and age, but not estrogen replacement, regulate N-methyl-d-aspartate receptor mRNA levels in the hippocampus of female rats. Exp Neurol. 2001b;170:345–356. - PubMed
- Alves SE, Weiland NG, Hayashi S, McEwen BS. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. - PubMed
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118:169–171. - PubMed
- Belisle S, Bellabarba D, Lehoux J-G. Hypothalamic-pituitary axis during reproductive aging in mice. Mech Ageing Dev. 1990;52:207–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous