A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat - PubMed (original) (raw)
A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat
Daniel A Clayton et al. J Neurosci. 2002.
Abstract
Aged rats are known to have deficits in spatial learning behavior in the Morris water maze. We have found that aged rats also have deficits in NR2B protein expression and that the protein expression deficit is correlated with their performance in the Morris water maze. To test whether this NR2B deficit was sufficient to account for the behavioral deficit, we used antisense oligonucleotides to specifically knock down NR2B subunit expression in the hippocampus of young rats. NR2B antisense treatment diminished NMDA receptor responses, abolished NMDA-dependent long-term potentiation (LTP), and impaired spatial learning. These data demonstrate the important role of NR2B in LTP and learning and memory and suggest a role for reduced NR2B expression in age-related cognitive decline.
Figures
Fig. 1.
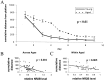
Spatial learning correlates with hippocampal expression of NR2B: training trials. A, Spatial learning, as assessed by three trials per day for 10 d in a Morris water maze hidden platform paradigm, reveals a significant age-related deficit in cumulative distance from platform (number of animals: aged,n = 8 at 16 months, 24 at 22 months, and 8 at 23 months; young, n = 14 at 6 months;p = 0.003 by repeated measures ANOVA). Distance units shown are in a linear, calibrated arbitrary tracking unit.B, Regression analysis between performance in the Morris Water maze on days 3 and 4 and expression of the NR2B subunit reveals a significant association across animals in all age groups (p = 0.0003;_R_2 = 0.23). Distance units shown are in a linear, calibrated arbitrary tracking unit.C, Regression analysis between performance in the Morris Water maze on days 3 and 4 and expression of the NR2B subunit reveals a significant association across animals in the aged group (16, 22, and 23 months; p = 0.005;_R_2 = 0.20). Distance units shown are in a linear, calibrated arbitrary tracking unit.
Fig. 2.
Spatial learning correlates with hippocampal expression of NR2B: probe trials. A, Spatial learning, as assessed by probe trials every other day during a 10 d, three trial per day training paradigm in a Morris water maze with a hidden platform, reveals a significant age-related increase in the average distance from expected platform (number of animals: aged,n = 8 at 16 months, 24 at 22 months, and 8 at 23 months; young, n = 14 at 6 months;p < 0.01 by repeated measures ANOVA). Distance units shown are in a linear, calibrated arbitrary tracking unit.B, Regression analysis between probe performance in the Morris Water maze on day 4 (average distance) and expression of the NR2B subunit reveals a significant association across animals in all age groups (p < 0.01;_R_2 = 0.25). Distance units shown are in a linear, calibrated arbitrary tracking unit. C, Regression analysis between performance in the Morris Water maze on day 4 (average distance) and expression of the NR2B subunit reveals a significant association across animals in the aged group (16, 22, and 23 months; p < 0.05;_R_2 = 0.19). Distance units shown are in a linear, calibrated arbitrary tracking unit.D, Spatial learning, as assessed by probe trials every other day during a 10 d, three trial per day training paradigm in a Morris water maze with a hidden platform, reveals a significant age-related deficit in the numbers of platform crossings (number of animals: aged, n = 8 at 16 months, 24 at 22 months, and 8 at 23 months, young, n = 14 at 6 months; p < 0.01 by repeated measures ANOVA). Distance units shown are in a linear, calibrated arbitrary tracking unit.
Fig. 3.
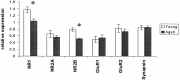
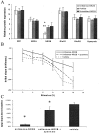
Age-related deficits in the expression of NMDA receptor subunits. Relative expression of NR2B protein between young and aged groups reveals a significant age-related decrease [37.5% reduction aged/young; number of animals: n = 38/14;p = 0.002, ANOVA with Fisher's protected least significant difference (PLSD) post hoc test]. A significant age-related decrease in NR1 levels is also found (25.2% reduction aged/young; number of animals: n = 38/14;p = 0.01, ANOVA with Fisher's PLSD post hoc test) that is not found in the relative expression of NR2A, GluR1, GluR2, or synapsin (p = 0.35;p = 0.44; p = 0.72;p = 0.98, respectively). _Asterisks_designate significant (<0.05) p values.
Fig. 4.
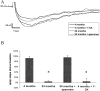
Age-related NMDA receptor functional deficits.A, Representative traces from pharmacologically isolated NMDA EPSPs recorded from Fischer 344 rats at 4 and 24 months of age revealing a deficit in aged responses (36.2% reduction 24/4 months; number of animals: n = 6; p = 0.02). This decline in the NMDA EPSP can be reproduced in young animals by treatment of the NMDA response with 6 μ
m
7-CK (45.3% reduction treated/untreated; number of animals: n = 6; p = 0.005) and can be ameliorated in aged animals by 100 μ
m
spermine (3.2% reduction 24 month spermine/4 months; number of animals: n = 6;p = 0.99). Responses shown are at the same stimulus intensity at the midrange of our standard I–O response curves.B, HFS-induced LTP measured 30 min after induction in the presence of 10 μ
m
nifedipine reveals a significant age-dependent decline 24 months (94.8% reduction 24/4 months, number of animals: n = 6; p = 0.0008) that is reproduced in young animals by the addition of 6 μ
m
7-CK (92.4% reduction treated/untreated; number of animals: n = 6; p = 0.003) and is ameliorated in aged animals by the addition of 100 μ
m
spermine (103% 24 month spermine/4 months; number of animals:n = 6; p = 1). All statistics performed by ANOVA with Fisher's PLSD post hoc test. *Significance (p < 0.05) compared with 4 month group.
Fig. 5.
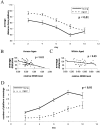
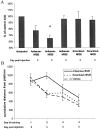
Effect of antisense-NR2B treatment.A, Injection of antisense-NR2B oligonucleotides leads to a selective hippocampal loss of NR2B protein at 3 d after injection (63% reduction antisense/scrambled; number of animals:n = 16/8; p = 0.002; 59% reduction antisense/vehicle; number of animals: n = 16/16; p = 0.001). No significant changes were seen in the expression of NR1, NR2A, GluR1, GluR2, or synapsin (all_p_ > 0.50, statistics by ANOVA with Fisher's PLSD_post hoc_ test within group, Bonferroni adjusted between groups). *Significance to vehicle treatment; **significance to scrambled treatment. B, NMDA receptor responses are diminished in slices from antisense-NR2B-treated animals relative to control (number of animals: n = 6;p = 0.04), and this difference can be ameliorated by treatment with 100 μ
m
spermine (number of animals:n = 6; p = 0.25, statistics by repeated measures ANOVA). C, Antisense-NR2B treatment leads to a decrease in the amount of HFS-induced LTP measured 30 min after induction (93.2% reduction antisense/vehicle;n = 6; p = 0.003), which can be ameliorated by 100 μ
m
spermine (70.8% reduction antisense/antisense and spermine; number of animals:n = 6; p = 0.008, statistics by ANOVA with Fisher's PLSD post hoc test). *Significance to antisense treatment.
Fig. 6.
Time dependent antisense-NR2B effects.A, Antisense-NR2B injection leads to a time-dependent decrease in the amount of hippocampal NR2B protein that is most significant on day 3 after injection (35% reduction antisense-NR2B/vehicle; number of animals: n = 16;p = 0.002). Scrambled-NR2B controls do not show a significant decrease in the amount of NR2B protein on either day 3 or day 4. B, Spatial learning performance, assessed by four trials per day for 4 d in a Morris water maze hidden platform task, shows a significant time-dependent difference between antisense-NR2B-treated animals and both vehicle- and scrambled-NR2B-treated controls (p = 0.058 by repeated measures ANOVA) that is most pronounced on day 2 of training, which corresponds to day 3 after injection (p = 0.02, antisense-NR2B/scrambled-NR2B;p = 0.035, antisense-NR2B/vehicle, by ANOVA with Fisher's PLSD post hoc test). Distance units shown are in a linear, calibrated arbitrary tracking unit.
Similar articles
- N-methyl-D-aspartate receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial learning.
Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR. Nihei MK, et al. Neuroscience. 2000;99(2):233-42. doi: 10.1016/s0306-4522(00)00192-5. Neuroscience. 2000. PMID: 10938429 - Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats.
Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Tombaugh GC, et al. J Neurosci. 2002 Nov 15;22(22):9932-40. doi: 10.1523/JNEUROSCI.22-22-09932.2002. J Neurosci. 2002. PMID: 12427850 Free PMC article. - Testing the NMDA, long-term potentiation, and cholinergic hypotheses of spatial learning.
Cain DP. Cain DP. Neurosci Biobehav Rev. 1998 Mar;22(2):181-93. doi: 10.1016/s0149-7634(97)00005-5. Neurosci Biobehav Rev. 1998. PMID: 9579310 Review. - [Endogenous formaldehyde regulates memory].
Fei XC, Tong ZQ. Fei XC, et al. Sheng Li Xue Bao. 2020 Aug 25;72(4):463-474. Sheng Li Xue Bao. 2020. PMID: 32820309 Review. Chinese.
Cited by
- Methamphetamine-induced short-term increase and long-term decrease in spatial working memory affects protein Kinase M zeta (PKMζ), dopamine, and glutamate receptors.
Braren SH, Drapala D, Tulloch IK, Serrano PA. Braren SH, et al. Front Behav Neurosci. 2014 Dec 18;8:438. doi: 10.3389/fnbeh.2014.00438. eCollection 2014. Front Behav Neurosci. 2014. PMID: 25566006 Free PMC article. - Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors.
Kochlamazashvili G, Bukalo O, Senkov O, Salmen B, Gerardy-Schahn R, Engel AK, Schachner M, Dityatev A. Kochlamazashvili G, et al. J Neurosci. 2012 Feb 15;32(7):2263-75. doi: 10.1523/JNEUROSCI.5103-11.2012. J Neurosci. 2012. PMID: 22396402 Free PMC article. - The role of the N-methyl-D-aspartate receptor in Alzheimer's disease: therapeutic potential.
Doraiswamy PM. Doraiswamy PM. Curr Neurol Neurosci Rep. 2003 Sep;3(5):373-8. doi: 10.1007/s11910-003-0019-8. Curr Neurol Neurosci Rep. 2003. PMID: 12914679 Review. - Circuit-specific changes in D-serine-dependent activation of the N-methyl-D-aspartate receptor in the aging hippocampus.
Labarrière M, Thomas F, Dutar P, Pollegioni L, Wolosker H, Billard JM. Labarrière M, et al. Age (Dordr). 2014;36(5):9698. doi: 10.1007/s11357-014-9698-0. Epub 2014 Aug 20. Age (Dordr). 2014. PMID: 25138794 Free PMC article. - Memory suppressor genes: Modulating acquisition, consolidation, and forgetting.
Noyes NC, Phan A, Davis RL. Noyes NC, et al. Neuron. 2021 Oct 20;109(20):3211-3227. doi: 10.1016/j.neuron.2021.08.001. Epub 2021 Aug 26. Neuron. 2021. PMID: 34450024 Free PMC article. Review.
References
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-d-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001;432:230–243. - PubMed
- Audinat E, Lambolez B, Rossier J, Crepel F. Activity-dependent regulation of N-methyl-d-aspartate receptor subunit expression in rat cerebellar granule cells. Eur J Neurosci. 1994;6:1792–1800. - PubMed
- Audinat E, Lambolez B, Rossier J. Functional and molecular analysis of glutamate-gated channels by patch-clamp and RT-PCR at the single cell level. Neurochem Int. 1996;28:119–136. - PubMed
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical