Cellular immune responses against hepatitis C virus: the evidence base 2002 - PubMed (original) (raw)
Review
Cellular immune responses against hepatitis C virus: the evidence base 2002
S Ward et al. Clin Exp Immunol. 2002 May.
Abstract
Hepatitis C virus (HCV) is an RNA virus which is estimated to persistently infect about 170 million people worldwide. After acute infection, there is an initial period during which long-term outcome is decided. There is strong evidence that the cellular immune responses, involving both CD4+ and CD8+ T lymphocytes, are involved at this stage and it is their effectiveness which determines outcome. What is not understood is what determines their effectiveness. The most important component of this is likely to be some aspect of epitope selection, itself dictated by host MHC. Thus, to understand host immunity to HCV, we need to have a detailed understanding of the peptides involved in T lymphocyte responses. In this review, we discuss the peptide epitopes that have been identified so far, and their potential significance. We relate this to a scheme of host defence which may be useful for understanding natural and vaccine-induced immunity.
Figures
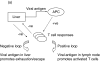
Fig. 1
A simple model for the role of T cell epitope selection in HCV outcome. (a) T cell responses are induced optimally in lymphoid tissue and migrate to the liver to suppress viral replication (positive loop). The liver environment, acccompanied by a high viral load serves to attenuate T cell responses (negative loop). High viral loads will also tend to promote escape. (b) An optimal ‘efficient’ response rapidly reduces viral load and thus accentuates the positive over the negative loop. The mechanism behind ‘efficiency’ is not well defined, but includes breadth, vigour, avidity (i.e. the ability to recognize infected cells sensitively), good effector function and targeting of ‘constrained’ epitopes. (c) An inefficient response fails to lower viral load rapidly and enters the negative loop. In this example the responses lack vigour or avidity, and are then attenuated further in the face of maintained viral loads. (d) An inefficient response fails to lower viral load early and allows for the emergence of escape variants. This will be promoted if the response is targeted at epitopes which can escape rapidly, or if the response is very highly focused.
Fig. 1
A simple model for the role of T cell epitope selection in HCV outcome. (a) T cell responses are induced optimally in lymphoid tissue and migrate to the liver to suppress viral replication (positive loop). The liver environment, acccompanied by a high viral load serves to attenuate T cell responses (negative loop). High viral loads will also tend to promote escape. (b) An optimal ‘efficient’ response rapidly reduces viral load and thus accentuates the positive over the negative loop. The mechanism behind ‘efficiency’ is not well defined, but includes breadth, vigour, avidity (i.e. the ability to recognize infected cells sensitively), good effector function and targeting of ‘constrained’ epitopes. (c) An inefficient response fails to lower viral load rapidly and enters the negative loop. In this example the responses lack vigour or avidity, and are then attenuated further in the face of maintained viral loads. (d) An inefficient response fails to lower viral load early and allows for the emergence of escape variants. This will be promoted if the response is targeted at epitopes which can escape rapidly, or if the response is very highly focused.
Fig. 1
A simple model for the role of T cell epitope selection in HCV outcome. (a) T cell responses are induced optimally in lymphoid tissue and migrate to the liver to suppress viral replication (positive loop). The liver environment, acccompanied by a high viral load serves to attenuate T cell responses (negative loop). High viral loads will also tend to promote escape. (b) An optimal ‘efficient’ response rapidly reduces viral load and thus accentuates the positive over the negative loop. The mechanism behind ‘efficiency’ is not well defined, but includes breadth, vigour, avidity (i.e. the ability to recognize infected cells sensitively), good effector function and targeting of ‘constrained’ epitopes. (c) An inefficient response fails to lower viral load rapidly and enters the negative loop. In this example the responses lack vigour or avidity, and are then attenuated further in the face of maintained viral loads. (d) An inefficient response fails to lower viral load early and allows for the emergence of escape variants. This will be promoted if the response is targeted at epitopes which can escape rapidly, or if the response is very highly focused.
Fig. 1
A simple model for the role of T cell epitope selection in HCV outcome. (a) T cell responses are induced optimally in lymphoid tissue and migrate to the liver to suppress viral replication (positive loop). The liver environment, acccompanied by a high viral load serves to attenuate T cell responses (negative loop). High viral loads will also tend to promote escape. (b) An optimal ‘efficient’ response rapidly reduces viral load and thus accentuates the positive over the negative loop. The mechanism behind ‘efficiency’ is not well defined, but includes breadth, vigour, avidity (i.e. the ability to recognize infected cells sensitively), good effector function and targeting of ‘constrained’ epitopes. (c) An inefficient response fails to lower viral load rapidly and enters the negative loop. In this example the responses lack vigour or avidity, and are then attenuated further in the face of maintained viral loads. (d) An inefficient response fails to lower viral load early and allows for the emergence of escape variants. This will be promoted if the response is targeted at epitopes which can escape rapidly, or if the response is very highly focused.
Similar articles
- Transmitted/Founder Viruses Rapidly Escape from CD8+ T Cell Responses in Acute Hepatitis C Virus Infection.
Bull RA, Leung P, Gaudieri S, Deshpande P, Cameron B, Walker M, Chopra A, Lloyd AR, Luciani F. Bull RA, et al. J Virol. 2015 May;89(10):5478-90. doi: 10.1128/JVI.03717-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740982 Free PMC article. - Broadening CD4+ and CD8+ T Cell Responses against Hepatitis C Virus by Vaccination with NS3 Overlapping Peptide Panels in Cross-Priming Liposomes.
Filskov J, Mikkelsen M, Hansen PR, Christensen JP, Thomsen AR, Andersen P, Bukh J, Agger EM. Filskov J, et al. J Virol. 2017 Jun 26;91(14):e00130-17. doi: 10.1128/JVI.00130-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28446674 Free PMC article. - Potent Anti-hepatitis C Virus (HCV) T Cell Immune Responses Induced in Mice Vaccinated with DNA-Launched RNA Replicons and Modified Vaccinia Virus Ankara-HCV.
Marín MQ, Pérez P, Ljungberg K, Sorzano CÓS, Gómez CE, Liljeström P, Esteban M, García-Arriaza J. Marín MQ, et al. J Virol. 2019 Mar 21;93(7):e00055-19. doi: 10.1128/JVI.00055-19. Print 2019 Apr 1. J Virol. 2019. PMID: 30674625 Free PMC article. - T cell responses in hepatitis C virus infection: historical overview and goals for future research.
Holz L, Rehermann B. Holz L, et al. Antiviral Res. 2015 Feb;114:96-105. doi: 10.1016/j.antiviral.2014.11.009. Epub 2014 Nov 26. Antiviral Res. 2015. PMID: 25433310 Free PMC article. Review. - The role of immune responses in the pathogenesis of hepatitis C virus infection.
Koziel MJ. Koziel MJ. J Viral Hepat. 1997;4 Suppl 2:31-41. doi: 10.1111/j.1365-2893.1997.tb00178.x. J Viral Hepat. 1997. PMID: 9429208 Review.
Cited by
- Acute hepatitis C in a chronically HIV-infected patient: evolution of different viral genomic regions.
Flichman D, Kott V, Sookoian S, Campos R. Flichman D, et al. World J Gastroenterol. 2003 Jul;9(7):1496-500. doi: 10.3748/wjg.v9.i7.1496. World J Gastroenterol. 2003. PMID: 12854149 Free PMC article. - Identifying immunologically-vulnerable regions of the HCV E2 glycoprotein and broadly neutralizing antibodies that target them.
Quadeer AA, Louie RHY, McKay MR. Quadeer AA, et al. Nat Commun. 2019 May 6;10(1):2073. doi: 10.1038/s41467-019-09819-1. Nat Commun. 2019. PMID: 31061402 Free PMC article. - Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection.
Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Dolganiuc A, et al. Gastroenterology. 2007 Nov;133(5):1627-36. doi: 10.1053/j.gastro.2007.08.003. Epub 2007 Aug 2. Gastroenterology. 2007. PMID: 17916356 Free PMC article. - Analysis of 'driver' and 'passenger' CD8+ T-cell responses against variable viruses.
Zafiropoulos A, Barnes E, Piggott C, Klenerman P. Zafiropoulos A, et al. Proc Biol Sci. 2004 Feb 7;271 Suppl 3(Suppl 3):S53-6. doi: 10.1098/rsbl.2003.0088. Proc Biol Sci. 2004. PMID: 15101418 Free PMC article. - TROLLOPE: A novel sequence-based stacked approach for the accelerated discovery of linear T-cell epitopes of hepatitis C virus.
Charoenkwan P, Waramit S, Chumnanpuen P, Schaduangrat N, Shoombuatong W. Charoenkwan P, et al. PLoS One. 2023 Aug 25;18(8):e0290538. doi: 10.1371/journal.pone.0290538. eCollection 2023. PLoS One. 2023. PMID: 37624802 Free PMC article.
References
- Pybus OG, Charleston MA, Gupta S, Rambaut A, Holmes EC, Harvey PH. The epidemic behavior of the hepatitis C virus. Science. 2001;292:2323–5. - PubMed
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. - PubMed
- Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. - PubMed
- Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–3. - PubMed
- Jessner W, Gschwantler M, Steindl-Munda P, et al. Primary interferon resistance and treatment response in chronic hepatitis C infection: a pilot study. Lancet. 2001;358:1241–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials