Administration of plasmids expressing interleukin-4 and interleukin-10 causes BALB/c mice to induce a T helper 2-type response despite the expected T helper 1-type response with a low-dose infection of Leishmania major - PubMed (original) (raw)
Administration of plasmids expressing interleukin-4 and interleukin-10 causes BALB/c mice to induce a T helper 2-type response despite the expected T helper 1-type response with a low-dose infection of Leishmania major
Kazuo Yamakami et al. Immunology. 2002 Apr.
Abstract
BALB/c mice are susceptible to developing an infection with Leishmania major as a result of a fatal T helper 2 (Th2)-type response. However, mice infected with a low dose of parasites are reported to be able to overcome the lesions associated with a T helper 1 (Th1)-type response. To clarify why a difference in the dose of parasites induces a difference in the polarization of the Th phenotype, we first attempted to measure cytokine production. Soon after infection, the mice given high doses of parasites produced elevated levels of both Th1 [interferon-gamma (IFN-gamma)] and Th2 [interleukin (IL)-4 and IL-10] cytokines. However, when assessed at 1 and 2 weeks after infection, no significant difference in the balance of Th1 and Th2 cytokines could be detected between mice infected with low or high doses of L. major. These results support the notion that the Th2 cytokine levels at an early phase of infection could be a key factor for the induction of a Th2 response. In order to assess the efficacy of Th2 cytokines, the mice infected with low doses of L. major were co-administered IL-4 plasmid and IL-10 plasmid. Consequently, the mice (which originally exhibited a Th1 response) showed progressive disease and developed a Th2 response. However, administration of these plasmids at 7 days postinfection could not alter the Th polarization. Furthermore, production of IL-12 from the spleen cells stimulated by L. major was suppressed in the presence of IL-4 and IL-10. These results strongly suggest that the susceptibility to L. major in BALB/c mice depends on the persistence of Th2 cytokine levels at an early phase of infection.
Figures
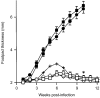
Figure 1
BALB/c mice infected with low doses of Leishmania major induced a protective response to the disease. Groups of mice (n = 6–10 per group) were infected in the left hind footpad with 1 × 102 (□), 1 × 103 (○), 1 × 104 (+), 1 × 105 (▪) or 1 × 106 (•) L. major, and C57BL/6 mice were also infected with 1 × 106 parasites (◊) as a control for the protective response. The weekly measurements of footpad thickness represent the average score ± standard error of the mean (SEM) of at least six mice per group. *P<0·001 for the group of mice infected with 1 × 102 L. major compared to mice infected with 1 × 106 parasites 10 weeks postinfection, based on Dunnett's _t_-test.
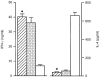
Figure 2
A T helper 1 (Th1)-type response in BALB/c mice, which had been infected with low doses of Leishmania major, was already established 4 weeks after infection. Single-cell suspensions from the popliteal lymph nodes of individual mice in the designated groups which had been infected with 1 × 102 (striped bars), 1 × 103 (dotted bars) or 1 × 106 (open bars) of L. major were prepared 4 weeks after infection. The cells were cultured in triplicate for 72 hr in the presence of soluble leishmanial antigen (SLA) and X-ray-irradiated splenocytes from syngenic mice as antigen-presenting cells (APC). After culture, the supernatants were collected and assayed for interferon-γ (IFN-γ) and interleukin (IL)-4 using an enzyme-linked immunosorbent assay (ELISA). Each bar represents the average ± standard error of the mean (SEM) for six mice per group. *P<0·001 compared with the group of mice infected with 1 × 106 parasites.
Figure 3
At an early stage of infection, cytokine profiles of mice that had received low doses of Leishmania major were comparable to those of mice that had received high doses, except for the quantitative levels. Single-cell suspensions from the popliteal lymph nodes were prepared from mice infected with low (1 × 102) or high (1 × 106) doses of L. major at 1 and 2 weeks postinfection, respectively, and the cells were stimulated with soluble leishmanial antigen (SLA) in the presence of antigen-presenting cells (APC). After culture for 72 hr, the supernatants were collected and cytokine levels determined using an enzyme-linked immunosorbent assay (ELISA). Each bar represents the average ± standard deviation (SD) of triplicate analyses.
Figure 4
Co-administration of pCAGGS interleukin (IL)-4 and pCAGGS IL-10 significantly increased lesion development in BALB/c mice infected with a low dose of Leishmania major. BALB/c mice (n = 6 per group) infected with a low dose of L. major were injected intradermally with IL-4 and IL-10 plasmids (10 µg/mouse) via the tail at 0 or 7 days postinfection. The untreated mice were also infected with low or high doses of parasites in the same manner as the respective controls. The weekly measurements of footpad thickness represent the average score ± standard error of the mean (SEM) of six mice per group. *P < 0·001 for values of group of mice co-administered IL-4 and IL-10 plasmids in comparison to the control mice which received the control plasmid (unmanipulated pCAGGS) on day 0.
Figure 5
Co-administration of interleukin (IL)-4 and IL-10 plasmids converted the cytokine profiles to T helper 2 (Th2) type in BALB/c mice in which the T helper 1 (Th1) type was expected to be induced with a low dose of Leishmania major. The single-cell suspensions from the popliteal lymph nodes of individual mice in the designated groups were prepared 15 weeks postinfection and then cultured in triplicate for 72 hr in the presence of soluble leishmanial antigen (SLA) and X-ray-irradiated splenocytes from syngenic mice as antigen-presenting cells (APC). The supernatants were collected and assayed for interferon-γ (IFN-γ) (striped bars) and IL-4 (dotted bars) using an enzyme-linked immunosorbent assay (ELISA). Each bar represents the average ± standard error of the mean (SEM) for six mice per group. *P < 0·001 compared to the group of control mice that received control (unmanipulated pCAGGS) plasmid.
Figure 6
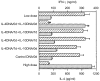
Co-administration of interleukin (IL)-4 and IL-10 plasmids enhanced the antigen-specific immunoglobulin (Ig)G1 antibody levels in BALB/c mice that had been infected with a low dose of Leishmania major. Soluble leishmanial antigen (SLA), specific serum IgG2a (striped bars) and IgG1 (dotted bars) levels were assessed by enzyme-linked immunosorbent assay (ELISA) 15 weeks after infection. The assays were performed at 1250-fold dilutions in triplicate for each serum sample. Serum from the BALB/c mice infected with a high dose of L. major was used as the standard for the IgG1-dominant profile. The values shown represent the mean ± standard error of the mean (SEM) of six mice per group. *P < 0·001 when comparing the levels of IgG1 and IgG2a, respectively, from the mice receiving plasmids versus those from the control mice which received empty plasmid (unmanipulated pCAGGS).
Figure 7
Combination of interleukin (IL)-4 and IL-10 suppressed the production of IL-12 in vitro. The single cell suspensions of splenocytes from naive BALB/c mice were stimulated with live Leishmania major in complete RPMI-1640 media for 24 hr. The cells were also stimulated with lipopolysaccharide (LPS) as the control. For the inhibitory efficacy of IL-4 and IL-10 on the production of IL-12, the cells were cultured in the presence of recombinant IL-4 and rIL-10. After culture, the supernatants were collected and the IL-12 (p40) levels were quantified using an enzyme-linked immunosorbent assay (ELISA). The data shown represent the mean ± standard deviation (SD) of triplicate experiments. *P < 0·001 in comparison to the controls which were stimulated with L. major in the absence of IL-4 and IL-10.
Similar articles
- Deletion of IL-4Ralpha on CD4 T cells renders BALB/c mice resistant to Leishmania major infection.
Radwanska M, Cutler AJ, Hoving JC, Magez S, Holscher C, Bohms A, Arendse B, Kirsch R, Hunig T, Alexander J, Kaye P, Brombacher F. Radwanska M, et al. PLoS Pathog. 2007 May 11;3(5):e68. doi: 10.1371/journal.ppat.0030068. PLoS Pathog. 2007. PMID: 17500591 Free PMC article. - A single intradermal administration of soluble leishmanial antigen and plasmid expressing interleukin-12 protects BALB/c mice from Leishmania major infection.
Yamakami K, Akao S, Sato M, Nitta Y, Miyazaki J, Tadakuma T. Yamakami K, et al. Parasitol Int. 2001 Jul;50(2):81-91. doi: 10.1016/s1383-5769(01)00070-8. Parasitol Int. 2001. PMID: 11438430 - Anti-leishmania effector functions of CD4+ Th1 cells and early events instructing Th2 cell development and susceptibility to Leishmania major in BALB/c mice.
Louis JA, Conceiçao-Silva F, Himmelrich H, Tacchini-Cottier F, Launois P. Louis JA, et al. Adv Exp Med Biol. 1998;452:53-60. doi: 10.1007/978-1-4615-5355-7_7. Adv Exp Med Biol. 1998. PMID: 9889959 Review.
Cited by
- Inhalative IL-10 attenuates pulmonary inflammation following hemorrhagic shock without major alterations of the systemic inflammatory response.
Kobbe P, Lichte P, Schreiber H, Reiss LK, Uhlig S, Pape HC, Pfeifer R. Kobbe P, et al. Mediators Inflamm. 2012;2012:512974. doi: 10.1155/2012/512974. Epub 2011 Oct 20. Mediators Inflamm. 2012. PMID: 22046081 Free PMC article. - Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity.
Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Mendez S, et al. J Exp Med. 2004 Jul 19;200(2):201-10. doi: 10.1084/jem.20040298. J Exp Med. 2004. PMID: 15263027 Free PMC article. - Site-dependent recruitment of inflammatory cells determines the effective dose of Leishmania major.
Ribeiro-Gomes FL, Roma EH, Carneiro MB, Doria NA, Sacks DL, Peters NC. Ribeiro-Gomes FL, et al. Infect Immun. 2014 Jul;82(7):2713-27. doi: 10.1128/IAI.01600-13. Epub 2014 Apr 14. Infect Immun. 2014. PMID: 24733090 Free PMC article. - Dectin-1 Positive Dendritic Cells Expand after Infection with Leishmania major Parasites and Represent Promising Targets for Vaccine Development.
Zimara N, Chanyalew M, Aseffa A, van Zandbergen G, Lepenies B, Schmid M, Weiss R, Rascle A, Wege AK, Jantsch J, Schatz V, Brown GD, Ritter U. Zimara N, et al. Front Immunol. 2018 Feb 26;9:263. doi: 10.3389/fimmu.2018.00263. eCollection 2018. Front Immunol. 2018. PMID: 29535708 Free PMC article. - Regulatory T cells and parasites.
Velavan TP, Ojurongbe O. Velavan TP, et al. J Biomed Biotechnol. 2011;2011:520940. doi: 10.1155/2011/520940. Epub 2011 Dec 29. J Biomed Biotechnol. 2011. PMID: 22262943 Free PMC article. Review.
References
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. - PubMed
- Launois O, Conceicao-Silva F, Himmerlich H, Parra-Lopez C, Tacchini-Cottier F, Louis JA. Setting in motion the immune mechanisms underlying genetically determined resistance and susceptibility to infection with Leishmania major. Parasite Immunol. 1998;20:223–30. - PubMed
- Constantinescu CS, Hondowicz BD, Elloso MM, Wysocka M, Trinchieri G, Scott P. The role of IL-12 in the maintenance of an established Th1 immune response in experimental leishmaniasis. Eur J Immunol. 1998;28:2227–33. <10.1002/%28sici%291521-4141%28199807%2928:07<2227::aid-immu2227>3.0.co;2-n>. - DOI - PubMed
- Heinzel FP, Rerko RM, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources