Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways - PubMed (original) (raw)
Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways
Lucinda F Reynolds et al. J Exp Med. 2002.
Abstract
Vav1 is a signal transducing protein required for T cell receptor (TCR) signals that drive positive and negative selection in the thymus. Furthermore, Vav1-deficient thymocytes show greatly reduced TCR-induced intracellular calcium flux. Using a novel genetic system which allows the study of signaling in highly enriched populations of CD4(+)CD8(+) double positive thymocytes, we have studied the mechanism by which Vav1 regulates TCR-induced calcium flux. We show that in Vav1-deficient double positive thymocytes, phosphorylation, and activation of phospholipase C-gamma1 (PLCgamma1) is defective. Furthermore, we demonstrate that Vav1 regulates PLCgamma1 phosphorylation by at least two distinct pathways. First, in the absence of Vav1 the Tec-family kinases Itk and Tec are no longer activated, most likely as a result of a defect in phosphoinositide 3-kinase (PI3K) activation. Second, Vav1-deficient thymocytes show defective assembly of a signaling complex containing PLCgamma1 and the adaptor molecule Src homology 2 domain-containing leukocyte phosphoprotein 76. We show that this latter function is independent of PI3K.
Figures
Figure 1.
Vav1 is required for normal TCR-driven calcium flux and IP3 generation. (A) Dot plots showing flow cytometric analysis of CD4 and CD8 expression on thymocytes from F5, Rag-1 −/−, β2m −/−, and Vav1 −/−, F5, Rag-1−/−, β2m−/− mice (Vav1 +/+ and Vav1 −/−) showing percentage of cells falling into a CD4+CD8+ DP gate. Histogram shows levels of TCR-β on DP thymocytes gated as on the dot plots. (B) Vav1 −/− or Vav1 +/+ thymocytes were preloaded with Indo-1, coated with anti-CD3ɛ, and the antibody cross-linked with goat anti–hamster antibody at the time indicated by the break in the calcium trace and the short vertical arrow. The graph shows the mean ratio of Indo-1 violet/blue fluorescence, a measure of intracellular [Ca2+], as a function of time. (C) Graphs showing mean levels of IP3 and PIP2 (±SEM) in DP thymocytes stimulated by cross-linking of CD3ɛ as above. Results in this and all other figures are representative of at least three independent experiments.
Figure 1.
Vav1 is required for normal TCR-driven calcium flux and IP3 generation. (A) Dot plots showing flow cytometric analysis of CD4 and CD8 expression on thymocytes from F5, Rag-1 −/−, β2m −/−, and Vav1 −/−, F5, Rag-1−/−, β2m−/− mice (Vav1 +/+ and Vav1 −/−) showing percentage of cells falling into a CD4+CD8+ DP gate. Histogram shows levels of TCR-β on DP thymocytes gated as on the dot plots. (B) Vav1 −/− or Vav1 +/+ thymocytes were preloaded with Indo-1, coated with anti-CD3ɛ, and the antibody cross-linked with goat anti–hamster antibody at the time indicated by the break in the calcium trace and the short vertical arrow. The graph shows the mean ratio of Indo-1 violet/blue fluorescence, a measure of intracellular [Ca2+], as a function of time. (C) Graphs showing mean levels of IP3 and PIP2 (±SEM) in DP thymocytes stimulated by cross-linking of CD3ɛ as above. Results in this and all other figures are representative of at least three independent experiments.
Figure 1.
Vav1 is required for normal TCR-driven calcium flux and IP3 generation. (A) Dot plots showing flow cytometric analysis of CD4 and CD8 expression on thymocytes from F5, Rag-1 −/−, β2m −/−, and Vav1 −/−, F5, Rag-1−/−, β2m−/− mice (Vav1 +/+ and Vav1 −/−) showing percentage of cells falling into a CD4+CD8+ DP gate. Histogram shows levels of TCR-β on DP thymocytes gated as on the dot plots. (B) Vav1 −/− or Vav1 +/+ thymocytes were preloaded with Indo-1, coated with anti-CD3ɛ, and the antibody cross-linked with goat anti–hamster antibody at the time indicated by the break in the calcium trace and the short vertical arrow. The graph shows the mean ratio of Indo-1 violet/blue fluorescence, a measure of intracellular [Ca2+], as a function of time. (C) Graphs showing mean levels of IP3 and PIP2 (±SEM) in DP thymocytes stimulated by cross-linking of CD3ɛ as above. Results in this and all other figures are representative of at least three independent experiments.
Figure 2.
Vav1 is required for normal tyrosine phosphorylation of PLCγ1 and Tec-family kinases. Vav1 +/+ and Vav1 −/− DP thymocytes were coated with anti-CD3ɛ antibody and then stimulated by cross-linking with a secondary antibody for the indicated times, or left unstimulated (0 s). In some cases total cell lysates were analyzed directly by immunoblotting with the indicated antibodies. In other cases the protein of interest was immunoprecipitated with specific antibody (IP Ab) and analyzed by immunoblotting with blotting antibodies (Blot Ab). Equal loading was always evaluated by reprobing immunoblots with antibodies specific for the protein being analyzed. Densitometry was performed as in Materials and Methods, and all signals were normalized to the maximum response (set to 100%). (A) Phosphorylation of PLCγ1 on tyrosine 783 (pY783) was analyzed by immunoblotting total cell lysates directly. Total tyrosine phosphorylation on PLCγ1 (p-PLCγ1) was analyzed by blotting PLCγ1 immunoprecipitates with anti-phosphotyrosine (anti-pTyr) antibodies. (B) Phosphorylation of ZAP-70 on tyrosine 319 (pY319) was analyzed by immunoblotting total cell lysates directly. Tyrosine phosphorylation on LAT and SLP-76 was analyzed by blotting immunoprecipitates with anti-pTyr antibodies. (C) Tyrosine phosphorylation on Itk and Tec was analyzed by blotting immunoprecipitates with anti-pTyr antibodies.
Figure 3.
Vav1 regulates association of PLCγ1 and SLP-76. Vav1 +/+ and Vav1 −/− DP thymocytes were stimulated and then analyzed by immunoprecipitation and immunoblotting as described in Fig. 2. (A) Immunoprecipitates of LAT were analyzed for the presence of PLCγ1, SLP-76, and Gads. (B) Immunoprecipitates of SLP-76 were analyzed for the presence of Gads. (C) Immunoprecipitates of PLCγ1 were analyzed for the presence of SLP-76.
Figure 3.
Vav1 regulates association of PLCγ1 and SLP-76. Vav1 +/+ and Vav1 −/− DP thymocytes were stimulated and then analyzed by immunoprecipitation and immunoblotting as described in Fig. 2. (A) Immunoprecipitates of LAT were analyzed for the presence of PLCγ1, SLP-76, and Gads. (B) Immunoprecipitates of SLP-76 were analyzed for the presence of Gads. (C) Immunoprecipitates of PLCγ1 were analyzed for the presence of SLP-76.
Figure 3.
Vav1 regulates association of PLCγ1 and SLP-76. Vav1 +/+ and Vav1 −/− DP thymocytes were stimulated and then analyzed by immunoprecipitation and immunoblotting as described in Fig. 2. (A) Immunoprecipitates of LAT were analyzed for the presence of PLCγ1, SLP-76, and Gads. (B) Immunoprecipitates of SLP-76 were analyzed for the presence of Gads. (C) Immunoprecipitates of PLCγ1 were analyzed for the presence of SLP-76.
Figure 4.
Vav1 is required for TCR-induced Akt phosphorylation and Rac1 activation. Vav1 +/+ and Vav1 −/− DP thymocytes were stimulated as described in Fig. 2. (A) Total cell lysates were analyzed for Akt phosphorylation by blotting with antibodies specific for phosphothreonine 308 (pT308) and phosphoserine 473 (pS473). (B) Rac1 activation was evaluated by pulling down active GTP-loaded Rac1 with a GST fusion protein containing the Rac binding domain of Pak1 (GST-Pak1-RBD) and blotting with anti-Rac1 antiserum. Equal quantities of Rac1 in the extracts were confirmed by immunoblotting a fraction of the total cell lysates taken before the GST-Pak1-RBD pulldown.
Figure 4.
Vav1 is required for TCR-induced Akt phosphorylation and Rac1 activation. Vav1 +/+ and Vav1 −/− DP thymocytes were stimulated as described in Fig. 2. (A) Total cell lysates were analyzed for Akt phosphorylation by blotting with antibodies specific for phosphothreonine 308 (pT308) and phosphoserine 473 (pS473). (B) Rac1 activation was evaluated by pulling down active GTP-loaded Rac1 with a GST fusion protein containing the Rac binding domain of Pak1 (GST-Pak1-RBD) and blotting with anti-Rac1 antiserum. Equal quantities of Rac1 in the extracts were confirmed by immunoblotting a fraction of the total cell lysates taken before the GST-Pak1-RBD pulldown.
Figure 5.
Vav1 regulates PLCγ1 activation via PI3K-dependent and -independent pathways. (A) Intracellular calcium concentration in Vav1 +/+ and Vav1 −/− DP thymocytes stimulated by cross-linking CD3ɛ was measured by Indo-1 fluorescence as described in Fig. 1 B. Cells were pretreated with the PI3K inhibitor Ly294002 as indicated. Cells not treated with inhibitor were preincubated with the appropriate concentration of the carrier DMSO. (B) Tyrosine phosphorylation of PLCγ1 and association of PLCγ1 with SLP-76 and Gads was determined using immunoprecipitates of PLCγ1 as described in Fig. 2 A and 3 C, respectively. Cells were pretreated with Ly294002 or with the carrier DMSO as indicated.
Figure 5.
Vav1 regulates PLCγ1 activation via PI3K-dependent and -independent pathways. (A) Intracellular calcium concentration in Vav1 +/+ and Vav1 −/− DP thymocytes stimulated by cross-linking CD3ɛ was measured by Indo-1 fluorescence as described in Fig. 1 B. Cells were pretreated with the PI3K inhibitor Ly294002 as indicated. Cells not treated with inhibitor were preincubated with the appropriate concentration of the carrier DMSO. (B) Tyrosine phosphorylation of PLCγ1 and association of PLCγ1 with SLP-76 and Gads was determined using immunoprecipitates of PLCγ1 as described in Fig. 2 A and 3 C, respectively. Cells were pretreated with Ly294002 or with the carrier DMSO as indicated.
Figure 6.
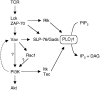
Cartoon of Vav-regulated pathways leading to PLCγ1 activation. TCR stimulation leads to the activation of the Lck and ZAP-70 kinases and the subsequent phosphorylation and activation of Vav. Vav regulates the activation of PI3K (as readout by Akt phosphorylation) possibly via Rac1, though there is no direct evidence for this. The dashed arrow represents the requirement for PI3K in the activation of Vav (54), leading to a potential positive feedback loop. Vav regulates PLCγ1 activation by at least two pathways, one PI3K-dependent, most likely through the activation of Itk and Tec. Secondly, Vav regulates the association between PLCγ1 and the SLP-76 and Gads adapters independently of PI3K activation. Rlk, another Tec-family kinase involved in PLCγ1 phosphorylation is activated in a PI3K-independent fashion since it does not have a PH domain (reference 61).
Similar articles
- Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1.
Reynolds LF, de Bettignies C, Norton T, Beeser A, Chernoff J, Tybulewicz VL. Reynolds LF, et al. J Biol Chem. 2004 Apr 30;279(18):18239-46. doi: 10.1074/jbc.M400257200. Epub 2004 Feb 5. J Biol Chem. 2004. PMID: 14764585 - Vav1: a key signal transducer downstream of the TCR.
Tybulewicz VL, Ardouin L, Prisco A, Reynolds LF. Tybulewicz VL, et al. Immunol Rev. 2003 Apr;192:42-52. doi: 10.1034/j.1600-065x.2003.00032.x. Immunol Rev. 2003. PMID: 12670394 Review. - Vav1 regulates phospholipase cgamma activation and calcium responses in mast cells.
Manetz TS, Gonzalez-Espinosa C, Arudchandran R, Xirasagar S, Tybulewicz V, Rivera J. Manetz TS, et al. Mol Cell Biol. 2001 Jun;21(11):3763-74. doi: 10.1128/MCB.21.11.3763-3774.2001. Mol Cell Biol. 2001. PMID: 11340169 Free PMC article. - The role of Tec family kinases in T cell development and function.
Lucas JA, Miller AT, Atherly LO, Berg LJ. Lucas JA, et al. Immunol Rev. 2003 Feb;191:119-38. doi: 10.1034/j.1600-065x.2003.00029.x. Immunol Rev. 2003. PMID: 12614356 Review.
Cited by
- Role of vav1 in the lipopolysaccharide-mediated upregulation of inducible nitric oxide synthase production and nuclear factor for interleukin-6 expression activity in murine macrophages.
Godambe SA, Knapp KM, Meals EA, English BK. Godambe SA, et al. Clin Diagn Lab Immunol. 2004 May;11(3):525-31. doi: 10.1128/CDLI.11.3.525-531.2004. Clin Diagn Lab Immunol. 2004. PMID: 15138177 Free PMC article. - Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways.
Houtman JC, Houghtling RA, Barda-Saad M, Toda Y, Samelson LE. Houtman JC, et al. J Immunol. 2005 Aug 15;175(4):2449-58. doi: 10.4049/jimmunol.175.4.2449. J Immunol. 2005. PMID: 16081816 Free PMC article. - TGFβ promotes mesenchymal phenotype of pancreatic cancer cells, in part, through epigenetic activation of VAV1.
Huang PH, Lu PJ, Ding LY, Chu PC, Hsu WY, Chen CS, Tsao CC, Chen BH, Lee CT, Shan YS, Chen CS. Huang PH, et al. Oncogene. 2017 Apr 20;36(16):2202-2214. doi: 10.1038/onc.2016.378. Epub 2016 Nov 28. Oncogene. 2017. PMID: 27893715 - Inverted signaling hierarchy between RAS and RAC in T-lymphocytes.
Zugaza JL, Caloca MJ, Bustelo XR. Zugaza JL, et al. Oncogene. 2004 Jul 29;23(34):5823-33. doi: 10.1038/sj.onc.1207768. Oncogene. 2004. PMID: 15184873 - Vav1 GEF activity is required for T cell mediated allograft rejection.
Haubert D, Li J, Saveliev A, Calzascia T, Sutter E, Metzler B, Kaiser D, Tybulewicz VL, Weckbecker G. Haubert D, et al. Transpl Immunol. 2012 Jun;26(4):212-9. doi: 10.1016/j.trim.2012.03.003. Epub 2012 Mar 21. Transpl Immunol. 2012. PMID: 22456277 Free PMC article.
References
- Kane, L.P., J. Lin, and A. Weiss. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242–249. - PubMed
- Henske, E.P., M.P. Short, S. Jozwiak, C.M. Bovey, S. Ramlakhan, J.L. Haines, and D.J. Kwiatkowski. 1995. Identification of VAV2 on 9q34 and its exclusion as the tuberous sclerosis gene TSC1. Ann. Hum. Genet. 59:25–37. - PubMed
- Schuebel, K.E., X.R. Bustelo, D.A. Nielsen, B.J. Song, M. Barbacid, D. Goldman, and I.J. Lee. 1996. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 13:363–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous