Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies - PubMed (original) (raw)
. 2002 May;160(5):1655-67.
doi: 10.1016/S0002-9440(10)61113-3.
Matthew P Frosch, Wei Ping Gai, Miguel Medina, Nutan Sharma, Lysia Forno, Tomoyo Ochiishi, Hideki Shimura, Ronit Sharon, Nobutaka Hattori, J William Langston, Yoshikuni Mizuno, Bradley T Hyman, Dennis J Selkoe, Kenneth S Kosik
Affiliations
- PMID: 12000718
- PMCID: PMC1850875
- DOI: 10.1016/S0002-9440(10)61113-3
Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies
Michael G Schlossmacher et al. Am J Pathol. 2002 May.
Abstract
Mutations in alpha-synuclein (alpha S) and parkin cause heritable forms of Parkinson disease (PD). We hypothesized that neuronal parkin, a known E3 ubiquitin ligase, facilitates the formation of Lewy bodies (LBs), a pathological hallmark of PD. Here, we report that affinity-purified parkin antibodies labeled classical LBs in substantia nigra sections from four related human disorders: sporadic PD, inherited alphaS-linked PD, dementia with LBs (DLB), and LB-positive, parkin-linked PD. Anti-parkin antibodies also detected LBs in entorhinal and cingulate cortices from DLB brain and alphaS inclusions in sympathetic gangliocytes from sporadic PD. Double labeling with confocal microscopy of DLB midbrain sections revealed that approximately 90% of anti-alpha S-reactive LBs were also detected by a parkin antibody to amino acids 342 to 353. Accordingly, parkin proteins, including the 53-kd mature isoform, were present in affinity-isolated LBs from DLB cortex. Fluorescence resonance energy transfer and immunoelectron microscopy showed that alphaS and parkin co-localized within brainstem and cortical LBs. Biochemically, parkin appeared most enriched in cytosolic and postsynaptic fractions of adult rat brain, but also in purified, alpha S-rich presynaptic elements that additionally contained parkin's E2-binding partner, UbcH7. We conclude that parkin and UbcH7 are present with alphaS in subcellular compartments of normal brain and that parkin frequently co-localizes with alpha S aggregates in the characteristic LB inclusions of PD and DLB. These results suggest that functional parkin proteins may be required during LB formation.
Figures
Figure 1.
Characterization of affinity-purified antibodies to human parkin. A: Schematic diagram of human parkin and five peptide antibodies (amino acid numbering according to Kitada et al 3 ). B: Dot blot analysis of selected proteins and parkin peptides. Bovine serum albumin, His-tagged Ub, human αS (10 ng each), and synthetic peptides (50 ng) were diluted in water and loaded horizontally. Membranes were probed with antibodies (Ab) as indicated (abs., absorbed with cognate peptide; sister lanes were mock-absorbed with noncognate parkin peptide). C: PAGE and Western blotting (WB) of recombinant αS and Ub (20 ng), soluble extracts from control brains (CNS, 40 μg) and lysates of SH-SY5Y cells stably expressing mycParkin (cell, 10 μg). Membranes were probed with antibodies to Ub, αS (syn-1), or parkin (HP6A, HP1A, HP1A abs., HP2A, and HP2A abs.), as indicated. Black arrowheads indicate relative position of monomeric Ub; white arrowheads identify proteolytic fragments of parkin. Asterisk denotes polyubiquitinated, high Mr protein smear in cell lysates. Note, anti-parkin antibodies fail to detect recombinant or endogenous αS and Ub proteins.
Figure 2.
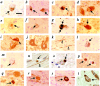
Immunohistochemistry with antibodies to parkin, αS, and UbcH7 in sections of the central and peripheral nervous system. Brainstem Lewy bodies (LB, arrows) are detected by HP2A (a and b), HP1A (c and d), HP7A (e and f), LB509 (g and h), and UbcH7 (t) in substantia nigra sections from PD (a, c, e, g, l, and t) and DLB (b, d, f, h, and m) but not control brain (i, j, and k). Open arrowhead depicts granular staining of perikaryon in a dopaminergic neuron (i); cells of cranial nerve III (j) and neurites (arrowheads) in control nigra (k) stained by HP1A. Competition with respective antigen [HP2A abs. (l) and HP1A abs. (m)] abolishes LB staining but not the appearance of surrounding melanin granules. In entorhinal (n and o) and cingulate cortex (p) sections from DLB brain (n, o, and p), cortical LBs are detected by HP2A (n), HP1A (o), and LB509 (p). In sympathetic gangliocytes from PD (q, r, and s), peripheral LBs are stained by HP2A (q), HP1A (r), and LB509 (s). Scale bar, 20 μm.
Figure 3.
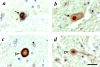
Immunohistochemistry with antibodies to αS and parkin in sections of substantia nigra from an αSA53T PD brain. Cytoplasmic Lewy bodies (a and b, arrows) and nonsomatic inclusions (c and d, arrowheads) are detected by LB509 (a and c) and HP2A (b and d). Scale bar, 20 μm.
Figure 4.
Immunohistochemistry with antibodies to parkin (HP2A in a, b, and d) and αS (LB509 in c and e) in sections of substantia nigra from a compound heterozygous, _parkin-_linked PD brain. Intracellular Lewy bodies (arrow) are seen at low power (a) and in adjacent, high power (original magnification, ×40). b to e are magnification images. Upper square in a denotes area of interest depicted in b and c, lower square images are depicted in d and e. Scale bar, 20 μm.
Figure 5.
Characterization of affinity-isolated cortical Lewy bodies from DLB brain. A: Schematic diagram of Percoll density gradient fractions that contained LB-rich material (LB-positive) isolated by magnetic beads coupled to sheep anti-αS antibody. B–D: PAGE and Western blotting (WB) of equal volumes of extracts from normal (LB-negative) and DLB (LB-positive) brain, and lysates (16 μg) of mycParkin-expressing transfected cells (293, CHO). Antibodies depicted are to the N-terminus of parkin (HP6A) and to αS (LB509, syn-1) (B); to parkin’s linker domain (HP1A) and to Ub (C); and to the in-between-RING domain of parkin (HP2A and HP2A abs.) (D). Note, in LB-positive material anti-parkin antibodies detect mature 53-kd parkin, an 11- to 12-kd fragment (open arrowheads), and gel-excluded high Mr parkin isoforms (asterisks). Anti-αS antibodies faintly recognize an ∼22-kd protein (black arrowhead). Brackets indicate oligomeric proteins.
Figure 6.
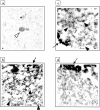
Light and electron microscopic localization of parkin protein in Lewy bodies (LB) from DLB cortex. a: LB staining (white arrowhead) in entorhinal cortex from DLB by immunohistochemistry with anti-parkin HP2A. b to d: LBs were isolated by magnetic beads (arrows) as in Figure 5A ▶ ; only segments of whole LBs are depicted. Antibody decoration of filamentous material by 12-nm gold particles (black arrowheads) using anti-αS (b) and anti-parkin HP2A (c), but not with preabsorbed HP2A (d).
Figure 7.
Mutational analysis of parkin’s cleavage in HEK293 cells. A: Diagram of two –NAxGG- motifs (underlined) in the sequence of human parkin (numbering according to Kitada et al 3 ), and of cDNA constructs encoding parkin proteins, as indicated. B: PAGE and autoradiogram of _in vitro_-translated cDNA constructs encoding full-length and truncated parkin proteins. C: PAGE and Western blotting (WB) of HEK293 cell lysates transfected with cDNA constructs encoding wild-type and mutant parkin proteins, as indicated. Note, generation of the 42-kd C-terminal parkin fragment was not abolished in parkin mutants, as detected by anti-parkin HP5A. Identical results were obtained using anti-parkin HP2A. Asterisks in B and C denote nonspecific background bands.
Figure 8.
a–e: Subcellular fractionation of adult rat brain generated total homogenate (HO), cytosol (CY), crude synaptosomes (CS), purified synaptosomes (PS), crude (TS) and purified (PRE) presynaptic terminal fractions, crude (TP), and purified (PSD) postsynaptic terminal fractions. Sixteen μg of each sample were loaded for PAGE. Western blots were probed with antibodies to parkin (HP2A, a), synaptophysin (b), αS (syn-1, c), PSD-95 (d), and UbcH7 (e). Lysates of HEK293 cells (293) expressing both mycParkin and untagged αS (a–d) or of Jurkat cells expressing UbcH7 (e) were analyzed in parallel. Note, that parkin, αS, and UbcH7 are present in the highly purified (PSD-95-negative) presynaptic fraction, PRE (vertical arrow).
Similar articles
- Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in lewy bodies in sporadic Parkinson's disease and in dementia with Lewy bodies.
Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Uéda K, Ikeda K, Kawai M. Arima K, et al. Brain Res. 1999 Oct 2;843(1-2):53-61. doi: 10.1016/s0006-8993(99)01848-x. Brain Res. 1999. PMID: 10528110 - [Parkinson's disease, dementia with Lewy bodies, multiple system atrophy and alpha-synuclein].
Iwatsubo T. Iwatsubo T. Rinsho Shinkeigaku. 1999 Dec;39(12):1285-6. Rinsho Shinkeigaku. 1999. PMID: 10791099 Japanese. - Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies.
Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Baba M, et al. Am J Pathol. 1998 Apr;152(4):879-84. Am J Pathol. 1998. PMID: 9546347 Free PMC article. - [Clinical and pathological study on early diagnosis of Parkinson's disease and dementia with Lewy bodies].
Orimo S. Orimo S. Rinsho Shinkeigaku. 2008 Jan;48(1):11-24. doi: 10.5692/clinicalneurol.48.11. Rinsho Shinkeigaku. 2008. PMID: 18386627 Review. Japanese.
Cited by
- Parkinson's disease.
Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA. Mhyre TR, et al. Subcell Biochem. 2012;65:389-455. doi: 10.1007/978-94-007-5416-4_16. Subcell Biochem. 2012. PMID: 23225012 Free PMC article. Review. - Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo.
Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, Boston H, Saftig P, Woulfe J, Feany MB, Myllykangas L, Schlossmacher MG, Tyynelä J. Cullen V, et al. Mol Brain. 2009 Feb 9;2:5. doi: 10.1186/1756-6606-2-5. Mol Brain. 2009. PMID: 19203374 Free PMC article. - Mitochondrial dysfunction in Parkinson's disease.
Keane PC, Kurzawa M, Blain PG, Morris CM. Keane PC, et al. Parkinsons Dis. 2011 Mar 15;2011:716871. doi: 10.4061/2011/716871. Parkinsons Dis. 2011. PMID: 21461368 Free PMC article. - Parkin promotes intracellular Abeta1-42 clearance.
Burns MP, Zhang L, Rebeck GW, Querfurth HW, Moussa CE. Burns MP, et al. Hum Mol Genet. 2009 Sep 1;18(17):3206-16. doi: 10.1093/hmg/ddp258. Epub 2009 May 29. Hum Mol Genet. 2009. PMID: 19483198 Free PMC article. - Imaging genetics approach to Parkinson's disease and its correlation with clinical score.
Kim M, Kim J, Lee SH, Park H. Kim M, et al. Sci Rep. 2017 Apr 21;7:46700. doi: 10.1038/srep46700. Sci Rep. 2017. PMID: 28429747 Free PMC article.
References
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Diiorio G, Golbe LI, Nussbaum RL: Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276:2045-2047 - PubMed
- Krüger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O: Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 1998, 18:106-108 - PubMed
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N: Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392:605-608 - PubMed
- Lücking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denefle P, Wood NW, Agid Y, Brice A: Association between early-onset Parkinson’s disease and mutations in the parkin gene. French Parkinson’s Disease Genetics Study Group. N Engl J Med 2000, 342:1560-1567 - PubMed
- Ujike H, Yamamoto M, Yamaguchi K, Kanzaki A, Takagi M, Kuroda S: Two cases of sporadic juvenile Parkinson’s disease caused by homozygous deletion of Parkin gene. No To Shinkei 1999, 51:1061-1064 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS02127/NS/NINDS NIH HHS/United States
- P50 NS038375/NS/NINDS NIH HHS/United States
- NS38375/NS/NINDS NIH HHS/United States
- P50 NS038372/NS/NINDS NIH HHS/United States
- NS38372/NS/NINDS NIH HHS/United States
- K08 NS002127/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases