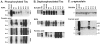Tau and alpha-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam - PubMed (original) (raw)
Tau and alpha-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam
Mark S Forman et al. Am J Pathol. 2002 May.
Abstract
Amyotrophic lateral sclerosis/parkinsonism-dementia complex (ALS/PDC) is a progressive neurodegenerative disorder of Chamorro residents of Guam and the Mariana Islands, characterized by abundant neuron loss and tau neurofibrillary pathology similar to that observed in Alzheimer's disease (AD). A variety of neurodegenerative diseases with tau pathology including ALS/PDC also have alpha-synuclein positive pathology, primarily in the amygdala. We further characterized the tau and alpha-synuclein pathology in the amygdala of a large series of 30 Chamorros using immunohistochemical and biochemical techniques. Tau pathology was readily detected in both affected and unaffected Chamorros. In contrast, alpha-synuclein pathology was detected in 37% of patients with PDC but not detected in Chamorros without PDC or AD. The alpha-synuclein aggregates often co-localized within neurons harboring neurofibrillary tangles suggesting a possible interaction between the two proteins. Tau and alpha-synuclein pathology within the amygdala is biochemically similar to that observed in AD and synucleinopathies, respectively. Thus, the amygdala may be selectively vulnerable to developing both tau and alpha-synuclein pathology or tau pathology may predispose it to synuclein aggregation. Furthermore, in PDC, tau and alpha-synuclein pathology occurs independent of beta-amyloid deposition in amygdala thereby implicating the aggregation of these molecules in the severe neurodegeneration frequently observed in this location.
Figures
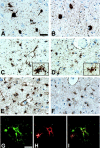
Figure 1.
Tau and GFAP positive pathology in the amygdala of Guam PDC patients. A and B: NFTs in the amygdala of PDC patients 21 (A) and 3 (B), immunostained with the phosphorylation-dependent tau mAb AT8. C and D: Numerous tau-positive astrocytes in the amygdala of PDC patients 10 (C) and 6 (D), immunostained with the mAb AT8. Inset shows high power magnification of tau-positive astrocytes with morphology resembling the hazy, granular astrocytes described by Oyanagi et al. E and F: Moderate to severe astrocytosis in the same amygdala of the patients illustrated in C and D, (patient 10, E; patient 6, F), immunostained with GFAP polyclonal antisera. G–I: Tau-positive hazy, granular astrocytes co-express GFAP. Double-labeling immunofluorescence of the amygdala of patient 5 stained with the mAb PHF1 (G, green) and GFAP polyclonal antisera (H, red). I represents an overlay of G and H. A–F: Scale bar, 40 μm. G–I: Scale bar, 20 μm.
Figure 2.
α-Synuclein-positive pathology in the amygdala of Guam PDC patients. A–G: Variable density of Lewy bodies and neuritic pathology in the amygdala of PDC patients immunostained with mAb Syn202. Patient 26 (A), diagnosed with AD, was assessed as having mild (1+) α-synuclein pathology; patients 3 (B) and 20 (C) were assessed as moderate; (2+) patient 10 (D) was assessed as having a marked (3+) density of α-synuclein pathology. E–G: Variable morphology of α-synuclein pathology including LBs that appear fragmented within the neuron (F, G). A–D: Scale bar, 40 μm. E–G: Scale bar, 20 μm.
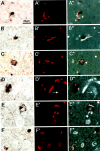
Figure 3.
Colocalization of LBs and NFTs in neurons of the amygdala of Guam PDC patients. Double-stained preparations for LBs and NFTs are as follows: A–F: Bright-field images of LBs immunostained with Syn202 using the ABC procedure and DAB as chromogen. A′-F′: Fluorescent images of Congo red stained NFTs in the same sections as shown in A–F. A″-F″: Fluorescent images after conversion to black and white superimposed onto the bright-field images. In A (patient 10), B (patient 3), and F (patient 6, upper right), three neurons are depicted in which the LB and NFT are located side by side. In C (patient 3), D and E (patient 21) and F (bottom left) the neurons show NFTs intermingled with LBs. The arrows in D′ and D″ point to extracellular NFTs which were not associated with LBs. Scale bar, 20 μm.
Figure 4.
Western blot analysis of soluble and insoluble tau and α-synuclein in the amygdala of Guam PDC patient. A: RIPA and insoluble (FA extractable) fractions were resolved by SDS-PAGE and immunoblotted with both phosphorylation-dependent (PHF1 and 12E8) and phosphorylation-independent (T14) tau antibodies as indicated. The insoluble tau from the Chamorro patients was composed of four major bands ranging from 60- to 72-kd similar to that observed in the non-Chamorro LBVAD control patient. In the formic acid extracts, the tau was also extensively aggregated. Molecular weight standards are indicated to the left of A. B: Aliquots of both soluble and insoluble (RIPA and formic acid extracts) were dephosphorylated with E. coli alkaline phosphatase, resolved by SDS-PAGE, and immunoblotted with T14. The soluble extracts were composed of all six tau isoforms that are normally expressed in adult brain. The RIPA and formic acid extracts from the Chamorro patients were also composed of all six tau isoforms similar to that observed in the non-Chamorro LBVAD patient. Recombinant tau isoforms (rTau) are indicated to the left of B. C: Soluble and formic acid fractions were resolved by SDS-PAGE and immunoblotted with Syn303. High molecular weight aggregates of insoluble synuclein was detected in two of three Chamorro patients with α-synuclein pathology detected by immunohistochemistry similar to that observed in the non-Chamorro LBVAD control. Non-aggregated α-synuclein is indicated by the arrowheads. The asterisks indicate proteins that are variably detected in both affected and normal control patients. Molecular weight standards are indicated to the right of C.
Similar articles
- Staging of cytoskeletal and beta-amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer's disease.
Mukaetova-Ladinska EB, Garcia-Siera F, Hurt J, Gertz HJ, Xuereb JH, Hills R, Brayne C, Huppert FA, Paykel ES, McGee M, Jakes R, Honer WG, Harrington CR, Wischik CM. Mukaetova-Ladinska EB, et al. Am J Pathol. 2000 Aug;157(2):623-36. doi: 10.1016/s0002-9440(10)64573-7. Am J Pathol. 2000. PMID: 10934165 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Brain-derived and in vitro-seeded alpha-synuclein fibrils exhibit distinct biophysical profiles.
Lee SS, Civitelli L, Parkkinen L. Lee SS, et al. Elife. 2024 Nov 25;13:RP92775. doi: 10.7554/eLife.92775. Elife. 2024. PMID: 39584804 Free PMC article. - Evaluation of Alpha-Synuclein and Tau Antiaggregation Activity of Urea and Thiourea-Based Small Molecules for Neurodegenerative Disease Therapeutics.
Ganegamage SK, Ademoye TA, Patel H, Alnakhala H, Tripathi A, Nguyen CCD, Pham K, Plascencia-Villa G, Zhu X, Perry G, Tian S, Dettmer U, Lasagna-Reeves C, Fortin JS. Ganegamage SK, et al. ACS Chem Neurosci. 2024 Nov 6;15(21):3915-3931. doi: 10.1021/acschemneuro.4c00282. Epub 2024 Oct 22. ACS Chem Neurosci. 2024. PMID: 39436010 Free PMC article. - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
- Tau and GSK-3β are Critical Contributors to α-Synuclein-Mediated Post-Stroke Brain Damage.
Mehta SL, Kim T, Chelluboina B, Vemuganti R. Mehta SL, et al. Neuromolecular Med. 2023 Mar;25(1):94-101. doi: 10.1007/s12017-022-08731-0. Epub 2022 Nov 30. Neuromolecular Med. 2023. PMID: 36447045 Free PMC article. - Neuronal clusterin expression is associated with cognitive protection in amyotrophic lateral sclerosis.
Gregory JM, Elliott E, McDade K, Bak T, Pal S, Chandran S, Abrahams S, Smith C. Gregory JM, et al. Neuropathol Appl Neurobiol. 2020 Apr;46(3):255-263. doi: 10.1111/nan.12575. Epub 2019 Aug 28. Neuropathol Appl Neurobiol. 2020. PMID: 31386770 Free PMC article. - Quantitative proteomics identifies surfactant-resistant alpha-synuclein in cerebral cortex of Parkinsonism-dementia complex of Guam but not Alzheimer's disease or progressive supranuclear palsy.
Yang W, Woltjer RL, Sokal I, Pan C, Wang Y, Brodey M, Peskind ER, Leverenz JB, Zhang J, Perl DP, Galasko DR, Montine TJ. Yang W, et al. Am J Pathol. 2007 Sep;171(3):993-1002. doi: 10.2353/ajpath.2007.070015. Epub 2007 Aug 3. Am J Pathol. 2007. PMID: 17675576 Free PMC article. - Investigating the Pathogenic Interplay of Alpha-Synuclein, Tau, and Amyloid Beta in Lewy Body Dementia: Insights from Viral-Mediated Overexpression in Transgenic Mouse Models.
Lim MJ, Boschen SL, Kurti A, Castanedes Casey M, Phillips VR, Fryer JD, Dickson D, Jansen-West KR, Petrucelli L, Delenclos M, McLean PJ. Lim MJ, et al. Biomedicines. 2023 Oct 22;11(10):2863. doi: 10.3390/biomedicines11102863. Biomedicines. 2023. PMID: 37893236 Free PMC article. - Tau Interacts with the C-Terminal Region of α-Synuclein, Promoting Formation of Toxic Aggregates with Distinct Molecular Conformations.
Dasari AKR, Kayed R, Wi S, Lim KH. Dasari AKR, et al. Biochemistry. 2019 Jun 25;58(25):2814-2821. doi: 10.1021/acs.biochem.9b00215. Epub 2019 Jun 7. Biochemistry. 2019. PMID: 31132261 Free PMC article.
References
- Murakami N: Parkinsonism-dementia complex on Guam. J Neurol 1999, 246(Suppl 2):II16-II18 - PubMed
- Oyanagi K, Wada M: Neuropathology of parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam: an update. J Neurol 1999, 246(Suppl 2):II19-II27 - PubMed
- Kurland LT: Amyotrophic lateral sclerosis and Parkinson’s disease complex on Guam linked to an environmental neurotoxin. Trends Neurosci 1988, 11:51-54 - PubMed
- Poorkaj P, Tsuang D, Wijsman E, Nemens E, Garruto R, Craig UK, Anderson L, Bird T, Plato CC, Weiderholt W, Galasko D, Schellenberg GD: Tau is a candidate gene for amyotropic lateral sclerosis-parkinsonism dementia complex. Arch Neurol 2001, 58:1871-1878 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous